Understand lithium carbonate and its applications
Lithium carbonate, an inorganic compound with the chemical formula Li2CO3, is a colorless monoclinic crystal or white powder. Density 2.11g/cm3, soluble in dilute acid, slightly soluble in water, solubility in cold water is greater than hot water, insoluble in alcohol and acetone.
Lithium carbonate is an important source for the preparation of various high-end physical products. According to factors such as processing difficulty, technological level and technical content, it can be divided into basic lithium products and high-end lithium products. Basic lithium products mainly include industrial-grade lithium carbonate and industrial-grade lithium hydroxide; high-end lithium products mainly include battery-grade lithium hydroxide, battery-grade lithium carbonate, pharmaceutical-grade lithium carbonate, and high-purity lithium carbonate.
Raw material of lithium carbonate
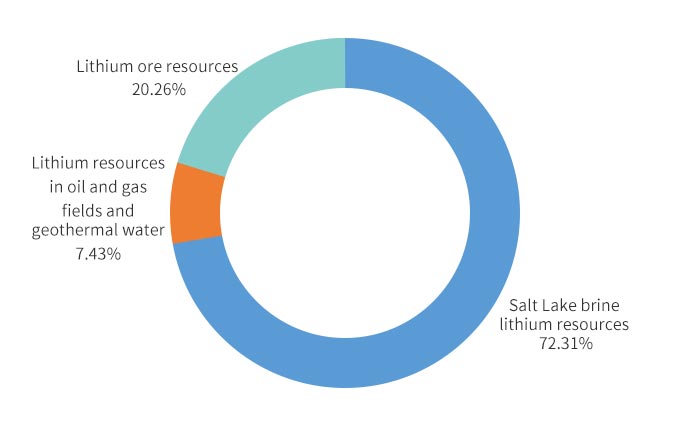
There are two main types of lithium in nature. About 70% of the world’s lithium exists in salt lakes, and about 30% comes from ore. According to USGS statistics, the world’s proven lithium reserves exceed 13.519 million tons (lithium metal); while the resources are as high as 39.78 million tons, equivalent to 210 million tons of lithium carbonate.
Lithium carbonate in the industry mainly refers to lithium ions and lithium ore. Lithium ions mainly exist in salt lake brine, underground brine and sea water. Lithium ore mainly refers to spodumene ore, spodumene, and lepidolite ore. The content of lithium in the earth’s crust is about 0.0065%, mainly distributed in South America, North America, Asia, Oceania and Africa.
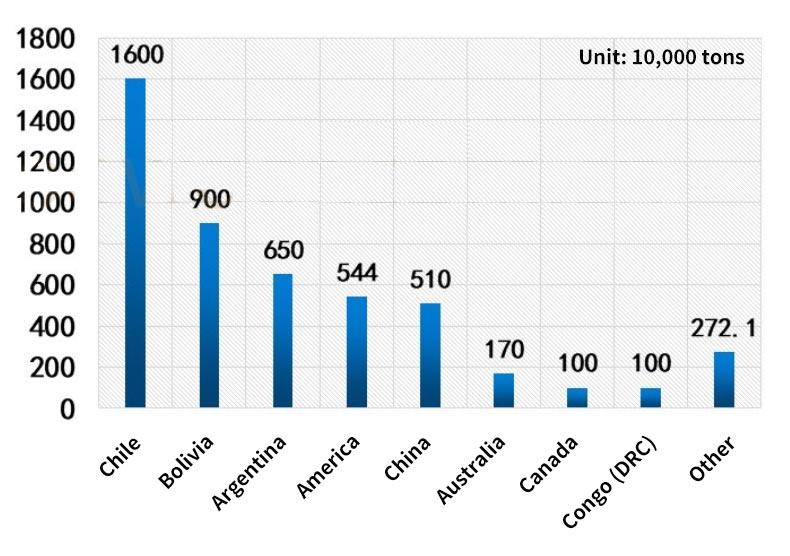
The top five countries with reserves of lithium resources account for 33.02% in Chile, 18.57% in Bolivia, 13.42% in Argentina, 11.23% in the United States, and 10.52% in China.
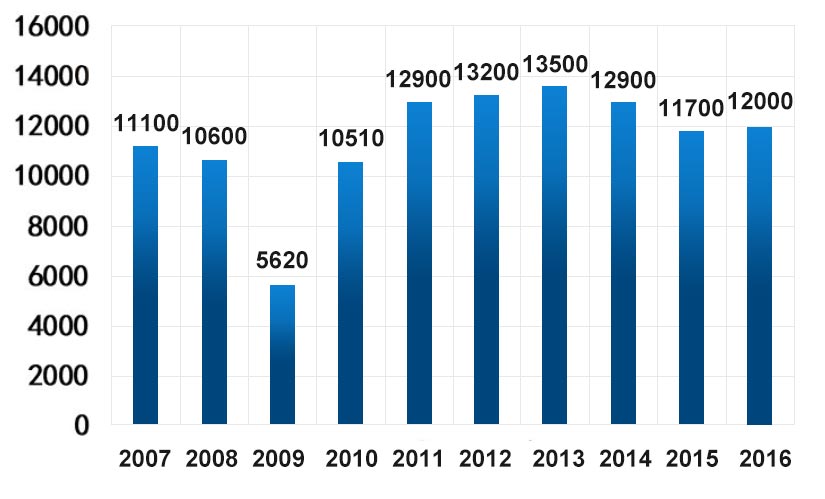
The global output of lithium mines is mainly contributed by Chile and Australia. The output of the two reached 26,300 tons in 2016, accounting for 75.14% of the total global output.
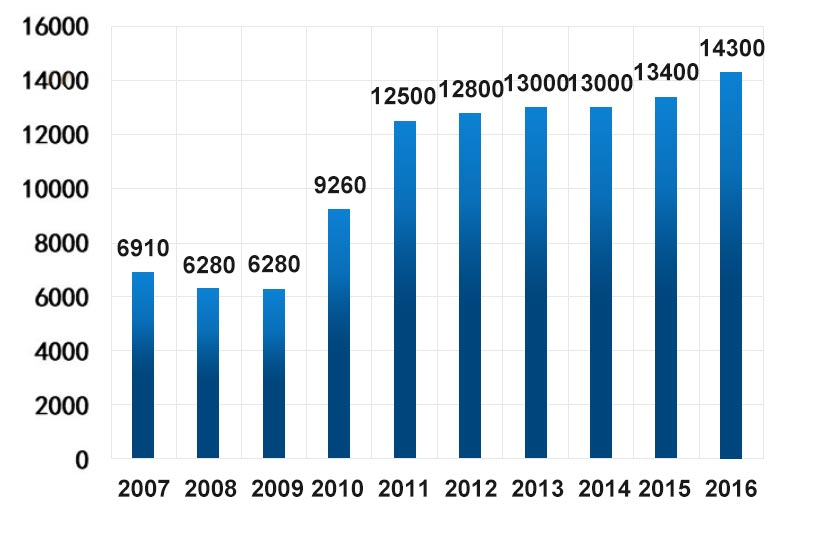
China’s lithium production is relatively low, but it is the largest consumer of lithium. It mainly imports most of the required lithium resources from Australia. Australia’s lithium mine output in the past ten years is as follows (unit: ton):
According to data released by the U.S. Geological Survey in 2015, China’s proven lithium resource reserves account for about 13% of the world’s total proven reserves. Among them, salt lake resources account for about 85% of the country’s total reserves, and ore resources account for about 15%. China’s lithium resources are mainly distributed in Qinghai. Tibet, Xinjiang, Sichuan, Jiangxi, Hunan and other provinces. Tibet and Qinghai are of salt lake brine type, while Xinjiang, Sichuan, Jiangxi and Hunan are of granite pegmatite or granite mineral type.
The preparation methods of lithium carbonate are mainly divided into two categories: ore lithium extraction method and salt lake brine method. Lithium extraction methods from ore mainly include limestone roasting, sulfuric acid method, and sulfate method. Salt lake brine methods mainly include powder adsorption method, solar pond concentration, solvent extraction method, calcining leaching method, and evaporation precipitation method.
Lithium Carbonate Application and Market
- Glass field
In glass manufacturing, lithium carbonate is mainly used in the production process of cathode picture tubes, heat-resistant glass, glass fiber and optical glass. Lithium carbonate can not only reduce the maturation and melting temperature of the glass, increase the density and strength of the glass, but also improve the viscosity and thermal expansion of the glass and many other important properties.

- Ceramics field
In the ceramic manufacturing process, adding an appropriate amount of lithium carbonate can not only increase the transparency and wear resistance of the product, but also reduce the expansion coefficient and melting temperature, thereby reducing fuel consumption and extending the life of the furnace.

- Medicine field
In the field of medicine, lithium carbonate can be used as a sleeping pill and tranquilizer, as well as anorexia nervosa, torticollis, arthritis, epilepsy, etc., and has become the drug of choice for mania.
- Non-ferrous metals
In the aluminum smelting industry, carbon materials containing 0.4% to 1.5% lithium carbonate are used as anodes instead of ordinary activated carbon materials, which can save 300 to 600 kW·h of electricity per ton of aluminum produced.
- Electrode material
Lithium carbonate is the most important product in lithium compounds. It is the main raw material for preparing metal Li, LiOH, LiBr, etc., not only for lithium ion batteries, but also for surface elastic wave component materials, lithium tantalate and lithium niobate.
Lithium carbonate is an indispensable industrial raw material. The downstream applications are: ceramics & glass 31%, batteries 23%, grease 9%, aluminum smelting 6%, refrigerant 6%, castings 4%, rubber 4%, pharmaceuticals 2%, the other 15%.
Article source: China Powder Network
