How to solve the problems of dispersion and large particles of talc powder used in coatings?

Talc, a commonly used functional filler in coatings, plays a crucial role in improving the mechanical properties of paint films, regulating rheology, and reducing costs. However, its poor dispersion stability and large particle size in coating systems directly affect the storage stability, application performance, and final film quality of the coatings.
1. Powder Pretreatment and Selection
Surface Modification: Surface-treated talc is preferred. Coating with silane coupling agents, titanate coupling agents, or stearic acid can significantly enhance its affinity with polymer bases, fundamentally reducing the tendency to agglomerate.
Particle Size and Distribution Control: Avoid using products with excessively wide original particle size distributions or containing ultra-coarse particles (>45μm). Narrowly distributed ultrafine talc (e.g., D50 of 5-15μm) generally has better dispersion potential.
2. Selection and Formulation of High-Efficiency Dispersants
The role of dispersants is to wet and break up agglomerates and maintain stability through steric hindrance or electrostatic repulsion.
(1) Aqueous Systems
Polyacrylates: General-purpose, providing electrostatic stability; pH and electrolyte stability must be considered.
Block Copolymers: Such as polyether-polyurethane, providing strong steric stability, firmly anchoring to hydrophobic surfaces (such as talc), and exhibiting good anti-flocculation effects, making them the first choice for solving large particle problems.
Compound Strategy: Wetting agents (such as acetylenic diols) are often compounded with high molecular weight dispersants to achieve a combination of rapid wetting and long-term stability.
(2) Solvent-Based Systems
Acidic/Alkaline Dispersants: These interact with the talc surface through anchoring groups; high molecular weight block copolymers are commonly used.
Key Evaluation Indicators: Molecular structure of the dispersant (anchoring groups and solvation chain length), dosage (optimal point determined by adsorption isotherms), and compatibility with the system.
Precise Optimization of Dispersion Process
The process is crucial for breaking agglomerates and achieving separation of primary particles.
(1) Pre-dispersion (wetting) stage
Using a high-speed disperser, slowly add talc powder to the solvent/base mixture at a low speed to ensure all powder is submerged in the liquid, forming a uniform paste. High speed should be avoided during this stage to prevent dust and air trapping.
A planetary mixer can effectively knead and mix the particles, especially effective in breaking up tightly packed agglomerates.
(2) High-efficiency grinding and dispersion stage
Sand mill/bead mill: The most effective equipment for eliminating micron-sized large particles.
Grinding media: Use smaller (e.g., 0.4-0.8mm zirconia beads) and higher hardness beads to increase collision frequency and shear force.
Rotor linear velocity: Maintained in a high shear range (typically >10m/s).
Number of passes: Typically 2-4 cycles are required depending on the initial particle size and target fineness. Online particle size monitoring allows for precise control of the endpoint.
Three-roll mill: Excellent for high-viscosity slurries and eliminating very small amounts of coarse particles (screen residue).
Quality Monitoring and Evaluation Methods
1. Particle Size Analysis
Laser Particle Size Analyzer: Monitors particle size distribution changes throughout the production process, focusing on D97, D100, and the tail trend of large particles. It is a core tool for judging dispersion effectiveness.
Hegmann Fineness Plate/Scraper Fineness Meter: Quickly and easily assesses the maximum particle size, suitable for on-site production control. The goal is to control the fineness below the target value (e.g., ≤25μm).
2. Microscopic Morphology Observation
The dispersion state and flaking of talc in the cross-section of the paint film are observed using scanning electron microscopy (SEM).
3. Stability Assessment
Storage Stability: After long-term standing, sedimentation, stratification, and the ease of redispersion are tested.
Thermal Storage Stability: Accelerates the testing of the system's resistance to flocculation.
4. Paint Film Performance Testing
Finally, the effect of dispersion on the improvement of paint film gloss, crack resistance, and scrub resistance is verified.
For high-end coating products, it is recommended to use a combination of "surface-modified talc powder + polymer block copolymer dispersant + sand milling process" to fundamentally and significantly improve the dispersion level of talc powder, eliminate harmful large particles, and thus give full play to its positive role in enhancing, reducing costs, and improving the performance of the coating film.
Three common modification methods for bentonite

Natural bentonite has extremely strong hydrophilicity and readily combines with water molecules in wastewater, making solid-liquid separation difficult after adsorption and limiting its application. Modified bentonite not only has much greater adsorption performance than natural bentonite but also expands its application range. Currently, there are many methods for modifying bentonite, commonly including activation modification, sodium modification, and modification with added modifiers.
I. Activation Modification
Activation modification involves activating natural bentonite using certain methods to enhance its adsorption performance. Commonly used activation methods include acidification activation, calcination activation, and inorganic salt activation.
(1) Acidification Activation
Acidification activation involves treating natural bentonite with acids of different concentrations, causing the Na+, Mg2+, K+, Ca2+, and other cations between the bentonite layers to be converted into soluble salts and dissolved, thereby weakening the bond energy between montmorillonite crystal layers, increasing the interlayer spacing, and forming a porous active material with a microporous mesh structure and a larger specific surface area. Commonly used acids include sulfuric acid and hydrochloric acid.
(2) Calcination Activation Method
The calcination activation method involves calcining bentonite at different temperatures to activate and modify it. When heated, bentonite loses interlayer water, bound water, and impurities in the pores, thereby increasing its specific surface area and porosity, reducing adsorption resistance caused by water films and impurities, and improving adsorption performance. A calcination temperature of 400-450℃ yields the best modification effect. High-temperature calcination activation modification requires strict control of calcination temperature and time; excessively high calcination temperatures or excessively long calcination times can easily lead to a decrease in bentonite activity.
(3) Salt Activation Method
The salt activation method typically uses halides of metal ions such as Na, Mg, Al, and Fe, as well as nitrates, as modifiers to treat bentonite. These metal cations balance the negative charge on the silicon-oxygen tetrahedra of bentonite. Because these cations have low valence and large radii, the interaction between them and the bentonite structural unit layers is weak, resulting in good ion exchange performance of bentonite.
II. Sodium Modification Method
The sodium modification method is mainly used for modifying calcium-based bentonite. Commonly used modification methods include suspension method, dry mixing method, wet stacking method, and wet extrusion method. Commonly used sodium modifiers include Na₂CO₃ and NaCl. The modification principle is through ion exchange, where Na⁺ replaces Ca²⁺ in the interlayer, creating a positive charge depletion. The Na⁺ adsorbed on the outer surface of the crystal and between the crystal layers then balances the negative charge.
Adsorption of Cd²⁺ using calcium-based bentonite and sodium-modified calcium-based bentonite showed that the saturated adsorption capacities of calcium-based bentonite and sodium-modified calcium-based bentonite were 2.96 mg/g and 8.45 mg/g, respectively. The adsorption capacity of sodium-modified calcium-based bentonite for Cd²⁺ was significantly greater than that of calcium-based bentonite.
III. Modification Method with Additive Modifiers
Modified bentonite obtained by the additive modifier method can be divided into three types: organic bentonite, cross-linked bentonite, and organic-cross-linked bentonite. Organic cross-linked bentonite involves introducing cationic surfactants with carbon chain lengths greater than 12 (such as quaternary ammonium salts like CTAB and CTAC) into the interlayer space of cross-linked bentonite for modification, resulting in organic cross-linked bentonite with larger pore sizes and further enhancing its adsorption performance.
Adding modifiers to bentonite can alter its specific surface area and increase interlayer spacing, thereby improving its adsorption performance. This is one of the main methods currently used for bentonite modification.
What are the types of powder grinding equipment, and what are their advantages and disadvantages?
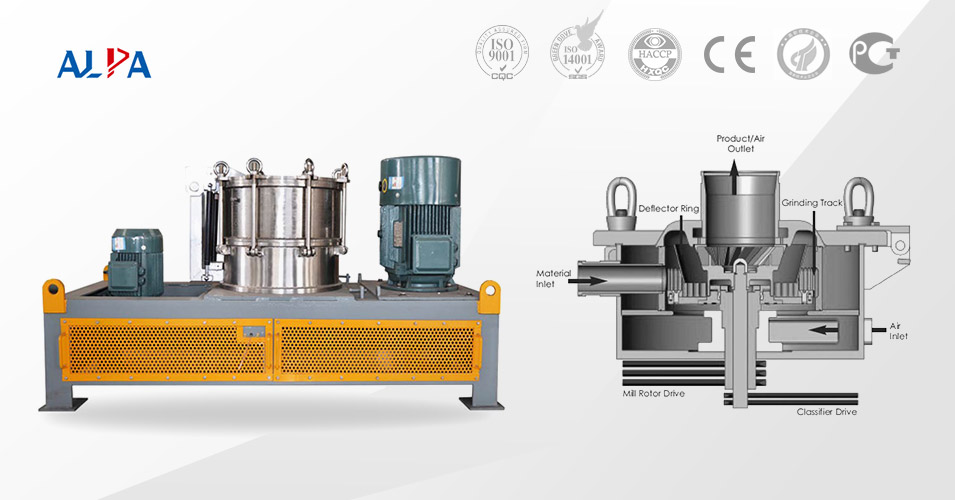
In industrial production, powder crushing is a fundamental and crucial process. Whether in the chemical, pharmaceutical, food, or mining industries, efficient crushing equipment is indispensable. Choosing the right crushing equipment can not only improve production efficiency but also optimize product quality. So, what are the common types of powder crushing equipment? What are their respective advantages and disadvantages? And in which scenarios are they suitable?
Powder crushing equipment comes in many varieties. Based on their working principles and applications, they can be broadly classified into the following categories:
1. Jaw Crusher
A jaw crusher is a common coarse crushing device that crushes materials through the squeezing action between a moving jaw and a stationary jaw. It has a simple structure and is suitable for materials with high hardness, such as ores and rocks.
Simple in structure, easy to maintain, and with a large processing capacity, it is suitable for coarse crushing of high-hardness materials.
The product particle size is relatively coarse, energy consumption is relatively high, and noise and vibration are relatively large.
2. Hammer Crusher
A hammer crusher uses high-speed rotating hammers to impact and crush materials. This equipment is suitable for medium-hardness and brittle materials, such as limestone and coal.
High crushing ratio, high output, suitable for medium-hard materials.
However, the hammers wear out relatively quickly, making it unsuitable for high-hardness materials, and it generates significant dust pollution.
3. Ball Mill
A ball mill crushes materials to the micron level through the impact and grinding action of steel or ceramic balls inside the mill. It is widely used in the mining, building materials, and chemical industries.
Fine particle size, suitable for various materials, can be processed dry or wet.
High energy consumption, large equipment size, grinding media easily abrades materials.
4. Air Jet Mill
An air jet mill uses a high-speed airflow to drive material collisions, achieving ultrafine grinding. This equipment is suitable for materials with high hardness and high purity, such as ceramic powder and pharmaceutical raw materials.
The product has uniform particle size and is pollution-free, making it suitable for ultrafine grinding of high-purity materials.
The equipment is costly and energy-intensive, making it suitable for small-batch production.
5. Vibratory Mill
A vibratory mill pulverizes materials through the combined action of high-frequency vibration and grinding media, suitable for fine grinding and mixing processes.
It boasts high grinding efficiency and a narrow particle size distribution, making it suitable for fine grinding and mixing.
However, the equipment has a complex structure and high maintenance costs.
6. Roller Mill
A roller crusher uses two opposing rotating rollers to compress materials, suitable for medium and fine crushing, and commonly used in the cement and metallurgical industries.
Particle size is controllable, energy consumption is low, and it is suitable for medium and fine crushing.
However, it has poor adaptability to moist and sticky materials, and the roller surface is prone to wear.
Selection of powder equipment
Mining Industry
Jaw crushers and ball mills are common choices in ore crushing and beneficiation. Jaw crushers are used for coarse crushing, while ball mills are used for fine grinding, ensuring the ore reaches the particle size required for subsequent processes.
Chemical Industry
Chemical raw materials typically require high purity and fineness. Air jet mills and vibratory mills can meet their needs for ultrafine powders while avoiding contamination.
Pharmaceutical and Food Industries
These industries have extremely high requirements for hygiene and safety. Air jet mills and vibratory mills are preferred due to their pollution-free and easy-to-clean characteristics. For example, air jet milling technology is often used for pulverizing pharmaceutical raw materials and food additives.
Building Materials Industry
The production of building materials such as cement and lime requires a large number of medium and fine crushing equipment. Roller crushers and hammer crushers are widely used due to their high output and low energy consumption.
New Materials Industry
With the development of new materials technology, the requirements for powder particle size and morphology are becoming increasingly stringent. Air jet mills and ball mills play an important role in the preparation of ceramic powders and metal powders.
Dust explosion prevention technology

Dust explosion refers to a chemical reaction in which combustible dust, mixed with an oxidizing gas (such as oxygen or air) to form a dust cloud within a confined space, rapidly burns in the presence of an ignition source, causing a rapid increase in temperature and pressure.
The conditions for a dust explosion generally include five factors:
(1) The presence of dust that can undergo an oxidation reaction with an oxidizing gas;
(2) The presence of an oxidizing gas;
(3) The presence of a high-temperature heat source sufficient to ignite the dust, i.e., an ignition source;
(4) The dust is suspended in the oxidizing gas, forming a dust cloud, and reaching the lower explosive limit;
(5) The dust cloud is confined within a relatively enclosed space.
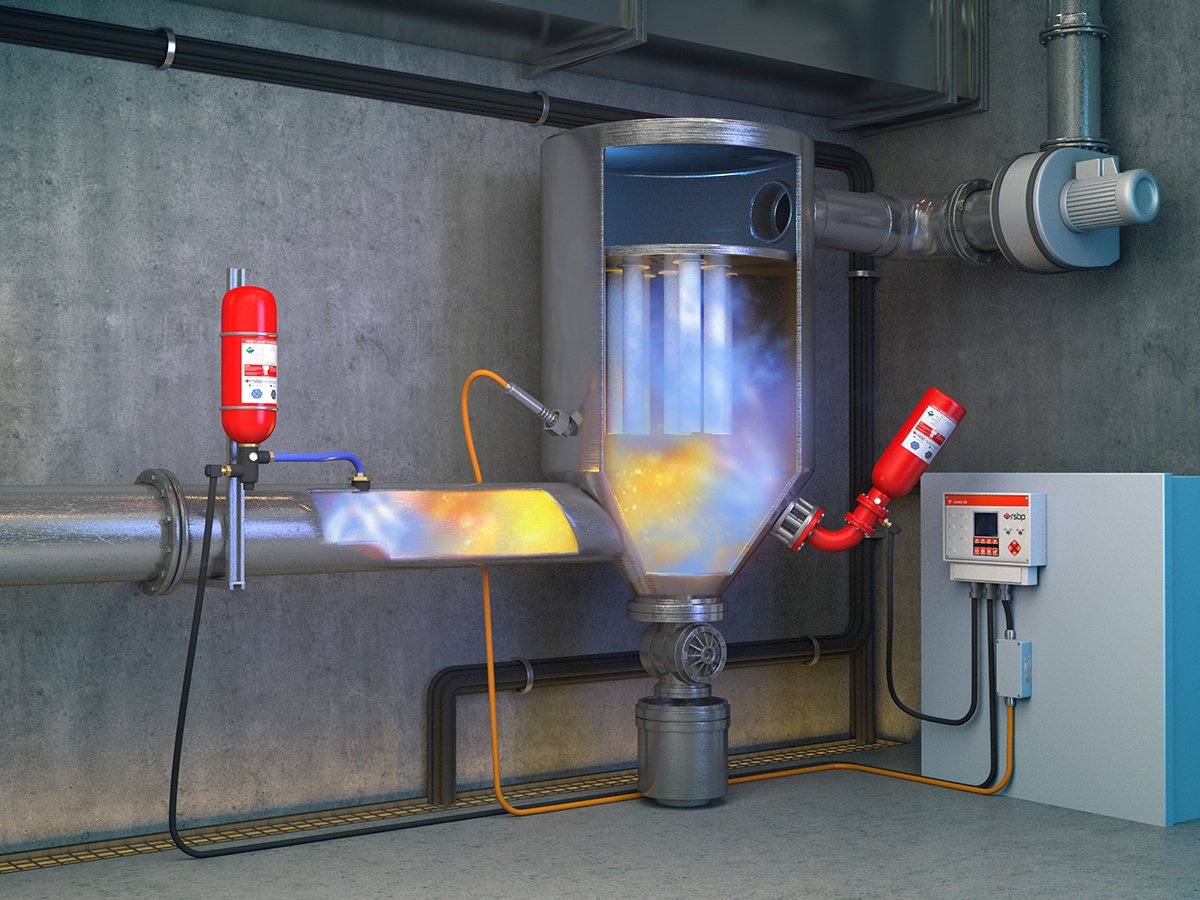
Dust Explosion Control Technologies
(1) Explosion-Proofing
This involves strengthening equipment and facilities to withstand a certain explosion pressure, thereby preventing equipment damage. For dust explosion-proof design, the design pressure is generally around 1.0 MPa, based on the maximum explosion pressure of approximately 0.9 MPa in a 20L sphere. However, most dust collectors currently have a pressure rating of only 30-50 kPa, so explosion venting needs to be considered.
(2) Explosion Venting
This involves installing pressure relief devices, such as rupture discs and pressure relief doors, on equipment and facilities to allow the rapid release of explosion pressure, reducing the peak pressure inside the equipment and thus minimizing the harm to equipment and personnel. Explosion venting devices need to be rationally designed and arranged according to the characteristics of the equipment and the working environment, taking into account the amplifying effect of internal turbulence in dust collectors and fluidized beds on dust explosions. It is worth noting that explosion venting can only reduce the maximum explosion pressure in the container and prevent container damage; it cannot prevent the flame of the dust explosion from spreading to other containers through pipes.
(3) Explosion Isolation
This involves setting up explosion isolation valves or suppressors to isolate the explosion area from other areas, preventing the explosion from affecting the entire system. This method is often used in complex piping and equipment systems to ensure that if an explosion occurs, the impact can be controlled within the smallest possible range.
(4) Explosion Suppression
Explosion suppression is also an effective method to reduce the explosion pressure inside a container. In the early stages of an explosion, a suppressant (such as a fire extinguishing agent or gas) is rapidly injected to inhibit the propagation and development of the explosion. Explosion suppression systems are usually equipped with sensors and automatic control devices that can activate the suppressant injection the moment an explosion signal is detected, thereby effectively controlling the explosion.
Preparation Process and Applications of Pharmaceutical-Grade Titanium Dioxide

In the medical system, titanium dioxide is used as a pharmaceutical white pigment due to its insolubility in acids and stable properties. It is used in capsules, coating powders, tablets, and medical devices, and is suitable for manufacturing opaque capsules, tablet film coatings, creams, pharmaceutical packaging materials, and pharmaceutical inks. In pharmaceutical formulations, titanium dioxide is an important component of protective coatings, improving the safety, efficacy, and quality of drugs over longer periods. Due to its ability to scatter light and absorb ultraviolet radiation, titanium dioxide extends shelf life and ensures drug stability by protecting active ingredients from UV light and thermal degradation.
Titanium dioxide is commonly used in the preparation of film-coating suspensions, sugar coatings, and gelatin capsules. It can also be mixed with other coloring agents and applied to topical preparations. It can also replace starch as an excipient, coating agent, coloring agent, and UV diluent in the preparation of coated tablets, pills, granules, capsules, and topical preparations. In color lakes, it is used as an opacifying agent to ensure uniform color, giving it a wide range of applications and promising market prospects.
The production of pharmaceutical-grade titanium dioxide requires strict standards for particle size distribution. This means that the crude product obtained from calcination must undergo a grinding process to ensure that the resulting titanium dioxide meets the requirements. During production, wet grinding technology is employed, using a sand mill to further refine the initially ground and slurried crude product. Zirconia beads and dispersants are used to ensure a uniform particle size distribution after grinding. Considering that the product will be used in the pharmaceutical field, the production process avoids adding additional chemical reagents for surface treatment to prevent the introduction of heavy metal ions. After grinding to the required fineness, the next step is washing, the purpose of which is to remove the salt treatment agents and dispersants added before calcination. The completion of washing can be monitored by an online conductivity electrode to detect impurity ions, or by using a 10% barium chloride solution to ensure the absence of sulfate ions. After successful washing, the material is dried in a drying oven to remove moisture and then sent to an air jet mill. No organic treatment agents are added, avoiding the toxicity to the human body caused by organic treatment agents, preventing interference with the active ingredients in the drug, and improving the effectiveness of the drug while also reducing production costs.
Medical-grade titanium dioxide has a wide range of applications. Considering various aspects such as medical examinations, medical diagnosis, and medical treatment, titanium dioxide is an effective method for medical diagnosis and treatment.
Based on the preparation methods of pharmaceuticals, this analysis examines the dosage specifications, preparation methods, and potential risk factors during the use of pharmaceutical products, and analyzes the limiting factors in the field of pharmaceuticals.
Based on key elements of hygiene and health bioscience assessment, the scope of application for medical-grade titanium dioxide is continuously expanded, and more extensive clinical evaluations and analyses of medical-grade titanium dioxide treatment are conducted.
What are the advantages of using barium sulfate in new energy vehicles?
The new energy industry, as one of the most promising strategic emerging industries of the 21st century, is experiencing unprecedented rapid development. With the increasing global emphasis on environmental protection and energy security, the drawbacks of traditional fossil fuels are becoming increasingly apparent, while new energy sources, with their clean, renewable, and low-carbon characteristics, are gradually becoming a key direction for global energy transformation.
Specific Applications of Barium Sulfate in New Energy Vehicles
Applications in Battery Materials: Barium sulfate plays an important role in the battery materials of new energy vehicles. Especially in lead-acid batteries, barium sulfate, as an additive, helps to improve the discharge performance and extend the service life of the battery. Specifically, barium sulfate can reduce the crystallization overpotential of lead sulfate, which facilitates the precipitation of lead sulfate crystals generated during discharge, thereby increasing battery capacity.
Applications in Coatings: In the body coatings of new energy vehicles, barium sulfate, as a filler, not only increases the opacity and whitening effect of the coating but also improves the adhesion and viscosity of the paint film, thus improving construction performance and coating quality. In addition, barium sulfate has excellent weather resistance, acid and alkali resistance, and gloss, which can enhance the overall performance of the coating.
Advantages of Barium Sulfate in New Energy Vehicles
Improved Battery Performance: By adding barium sulfate, the discharge capacity and cycle life of lead-acid batteries can be effectively improved, which is crucial for the long driving range and high energy density of new energy vehicles.
Enhanced Body Protection: The application of barium sulfate in body coatings not only improves the appearance quality of the vehicle but also enhances the body's resistance to harsh environments, such as ultraviolet rays and acid rain, thereby protecting the vehicle from damage.
Applications of Barium Sulfate in Other Fields and its Potential Impact on New Energy Vehicles
Applications in the Medical Field: Although primarily used in the medical field, some characteristics of barium sulfate (such as high biocompatibility and stability) may provide inspiration for the design of battery management systems (BMS) in new energy vehicles, especially in terms of material selection and safety.
Environmental Protection and Sustainability: As an environmentally friendly material, the low environmental impact of barium sulfate during its production and application is a positive signal for the new energy vehicle industry. With the global emphasis on sustainable development, the use of environmentally friendly materials such as barium sulfate helps to promote the green transformation of the new energy vehicle industry.
Barium sulfate plays an important role in the battery materials and body coatings of new energy vehicles, not only improving vehicle performance but also enhancing body protection. At the same time, its applications in other fields have provided reference and inspiration for its further development in new energy vehicles.
The wide applications of spherical alumina

Due to its larger surface area and uniform distribution compared to other morphologies, spherical alumina powder exhibits superior performance in practical applications compared to other shapes of alumina materials. It can be used not only in ceramics, catalysts and their carriers, but also in various fields such as grinding, polishing, and electronic devices.
Thermal Conductive Filler Field
With the advent of the information age, advanced electronic devices are becoming increasingly miniaturized, and the heat generated by these devices is increasing exponentially, placing many demands on system heat dissipation. Because alumina is widely available in the market, comes in many varieties, and is cheaper than other thermal conductive materials, and can be added in large quantities to polymer materials, it has a high cost-performance ratio. Therefore, most high-thermal conductivity insulating materials currently use alumina as a high-thermal conductivity filler.
Ceramics Field
Adding a certain amount of spherical alumina powder during the production of ceramics can significantly change the properties of the ceramics. The low-temperature brittleness of ceramics greatly affects their application range. Ceramic materials with added spherical alumina powder can be used to manufacture low-temperature ductile ceramics.
Grinding and Polishing Field
Compared with traditional granular or flake alumina, spherical alumina has better dispersibility and fluidity. Spherical alumina powder abrasives can be evenly distributed in the polished product, avoiding abnormal powder accumulation. Furthermore, the smooth surface of the particles prevents scratching the workpiece surface, thus improving the surface finish.
Electronic and Optical Materials Field
Spherical alumina has a wide range of applications in the electronic and optical fields. Using spherical alumina as a substrate and adding rare earth elements as activators, this method can produce red luminescent materials with better performance. Spherical alumina particles are uniform in size and evenly dispersed, exhibiting better luminescence performance compared to other shapes of alumina, and better determining the filling structure of the luminescent material.
Catalyst and Carrier Field
Because alumina has a large number of unsaturated chemical bonds on its surface and a large number of catalytic active centers, it exhibits high chemical activity. Moreover, spherical alumina has the advantages of low particle wear, long service life, and large specific surface area.
3D Printing Field
Spherical alumina is one of the most commonly used materials for 3D printing due to its high strength, high sphericity, and high-temperature resistance.
Surface Protective Coatings
The use of spherical alumina as a spray coating material is currently one of the research hotspots. This spray coating material not only provides protection for polymer materials, glass, metals, and alloys, but also extends the lifespan of stainless steel products such as kitchen cookware.
From all perspectives, fine alumina has become one of the new materials that my country needs to prioritize for development. With its widespread application in traditional fields and rapid penetration into emerging industries such as new energy vehicles and photovoltaic power generation, demand is constantly increasing, and the fine alumina industry has broad market prospects.
Five typical applications of talc powder

When talc's multiple powerful "superpowers" are unleashed in coatings, it can significantly improve material performance while substantially reducing product costs, leading to a comprehensive improvement in coating quality. Therefore, talc is widely used in various coating formulations.
Architectural Coatings
When talc powder is used in architectural coatings, it provides excellent brushability, gloss retention, and leveling properties. At the same time, the drying properties, tackiness, hardness, and corrosion resistance of the coating are significantly improved. It enhances the dry and wet hiding power, matting effect, crack resistance, and scrub resistance of the coating product, and can greatly improve the tinting strength of titanium dioxide, thus reducing product costs. In the use of materials for architectural coatings, talc is an indispensable component.
Industrial Coatings
Talc powder is widely used as a functional filler in various industrial coatings, especially in primer coatings for parts. Due to its good sanding and water resistance, talc powder can completely or partially replace primer fillers. When applied to steel structure coatings, talc powder effectively improves the sedimentation properties of the coating, the mechanical properties of the film, and recoatability. Many products, such as flash-drying primers and coatings for transportation vehicles, prioritize the use of talc powder.
Wood Coatings
Talc also holds a place in wood (furniture) coatings.
The application of talc powder in wood coatings is mainly in transparent primers and solid color topcoats. The low hardness characteristics of talc powder give the paint film good sandability, allowing for partial replacement of high-cost zinc stearate sanding agents. The refractive index of talc is similar to that of resin binders, giving the coating high transparency. This characteristic allows the natural texture of the substrate to be well displayed, and when used in matte topcoats, it can partially replace expensive matting agents.
When talc is used in wood coatings, it can maximize the charm of wooden furniture while satisfying people's pursuit of lifestyle and reducing living costs.
Anti-corrosion Coatings
Talc is still frequently seen in the field of anti-corrosion coatings. Talc's naturally stable lamellar structure increases the viscosity of the paint and provides a shielding effect to the paint film. While effectively preventing the penetration of corrosive media such as acids, alkalis, and salts, it also hinders the penetration of the primer on porous substrates, improving the sealing effect and sandability of the primer. These characteristics significantly improve the anti-corrosion performance of the paint film. In the field of anti-corrosion coatings, talc is a solid and reliable partner, worthy of trust.
Waterproof Coatings
As a filler in waterproof coatings, talc powder not only reduces volume shrinkage during coating curing, improves the wear resistance and adhesion of the coating, and reduces costs, but also gives the coating good storage stability and heat resistance.
More importantly, talc powder has a beneficial effect on the elastic elongation and tensile strength of waterproof coatings: within a certain range of addition, as the amount of talc powder filler increases, the elastic elongation and tensile strength of the waterproof coating both increase. This also means maximum protection for the coated object.
The application of talc in architectural coatings, industrial coatings, wood coatings, anti-corrosion coatings, and waterproof coatings is only a small part of its many application fields. As an inexpensive, non-renewable non-metallic mineral, talc also has wide applications in cosmetics, food, medicine, rubber, ceramics, textiles, printing and dyeing, and the electronics industry. It is believed that in the near future, with further research, humanity's understanding of talc will become increasingly profound, and talc will surely shine brightly in even broader fields.
How does barium sulfate contribute to the creation of high-quality coating materials?

Barium sulfate is highly favored primarily due to its exceptional filling capacity. This means that while maintaining paint film performance, it can effectively optimize formulation costs and is widely used in various fields, from industrial coatings to decorative paints.
More importantly, thanks to its small particle size, uniform distribution, large specific surface area, and excellent fluidity, barium sulfate exhibits very low abrasiveness during processing. This characteristic directly translates into production efficiency: it significantly reduces wear and tear on mixing, pumping, and spraying equipment, extending equipment lifespan and making the production process smoother and more economical.
This advantage is fully demonstrated in the application of automatic primer surface coatings. Even under high filling rate production requirements, barium sulfate ensures excellent stability and leveling properties of the paint slurry, resulting in exceptional uniformity and smoothness. This provides a flawless "canvas" for subsequent topcoat application, which is crucial for achieving efficient, automated, and high-quality coating.
Barium sulfate is far more than just a simple filler. It is a multifunctional additive that combines high filling capacity, low abrasion, and excellent leveling properties. Choosing it means selecting a reliable "foundation of quality" for your coatings, enhancing product performance while also ensuring efficient production.
Applications of advanced ceramic materials

Applications in High-Speed Aircraft
High-speed aircraft are strategic equipment that major military powers are vying to develop. Their supersonic flight and sharp structures lead to serious aerodynamic heating problems. The typical thermal environment for high-speed aircraft involves high temperatures and complex, harsh thermo-mechanical loads. Existing high-temperature alloys can no longer meet the requirements, leading to the emergence of ceramic matrix composites. In particular, SiCf/SiC composite ceramic materials have been widely used in hot structural components such as turbine blades, nozzle guide vanes, and turbine outer rings of aero-engines. Their composite material density is approximately 1/4 that of high-temperature alloys, resulting in significant weight reduction. Furthermore, they can operate at temperatures up to 1400°C, greatly simplifying cooling system design and enhancing thrust.
Applications in Lightweight Armor
Lightweight composite armor is crucial for maintaining the survivability of modern equipment. The development of ceramic fibers and fiber-reinforced ceramic matrix composites is fundamental to the application of lightweight composite armor. Currently, the main protective ceramic materials used include B4C, Al2O3, SiC, and Si3N4. Silicon carbide ceramics, with their excellent mechanical properties and cost-effectiveness, have become one of the most promising bulletproof ceramic materials. Their diverse applications in various armor protection fields, including individual soldier equipment, army armored weapons, armed helicopters, police and civilian special vehicles, give them broad application prospects. Compared to Al2O3 ceramics, SiC ceramics have a lower density, which is beneficial for improving equipment mobility.
Applications in Small Arms
Small arms, as an important component of weaponry, generally include pistols, rifles, machine guns, grenade launchers, and special individual equipment (individual rocket launchers, individual missiles, etc.). Their main function is to launch projectiles to the target area to kill or destroy enemy targets. The operating conditions of small arms include high temperature, low temperature, high altitude, humid heat, dust, rain, dust-rain, salt spray, and immersion in river water. Corrosion resistance is crucial. Currently, the main anti-corrosion processes for small arms include bluing, hard anodizing, ion-controlled penetration technology, diamond-like carbon coatings, and plasma nitriding. Especially for weapons and equipment used in marine environments, the requirement for corrosion resistance in salt spray environments for more than 500 hours poses a significant challenge to traditional coating treatments.
Applications in Gun Barrels
The gun barrel is a core component of projectile weapons. The internal structure of the gun barrel includes the chamber, the forcing cone, and the rifling, with the chamber and rifling connected by the forcing cone. Traditional gun barrels are generally made of high-strength alloy steel. During firing, the inside of the gun barrel is subjected to the combined effects of propellant gases and projectiles, leading to cracks and coating detachment on the inner wall of the barrel. The damage to the gun barrel's bore is a result of the repeated action of high-temperature, high-pressure, and high-velocity propellant gases and projectiles on the barrel wall. The forcing cone and the muzzle are usually the first parts to fail.
To improve gun barrel life, chromium plating of the bore is the most common method, but the oxidation resistance temperature of the chromium plating layer does not exceed 500°C. With the continuous increase in chamber pressure during firing and the exponential increase in gun barrel life requirements, the pressure and temperature borne by the gun barrel are also increasing. Utilizing the high hardness, high strength, and high-temperature chemical inertness of ceramics can effectively reduce gun barrel erosion and extend its service life.
Applications in Ammunition
The main components of ammunition are the warhead and the fuze. As the most direct component for causing damage, the warhead mainly consists of the casing, fragmentation elements, explosive charge, and fuze. Continuously improving the lethality of the warhead has always been a goal pursued in weapon development. Especially for area-effect grenades, the fragments produced by the warhead explosion are the terminal killing elements, and efficient fragmentation technology has always been a research challenge in this field.