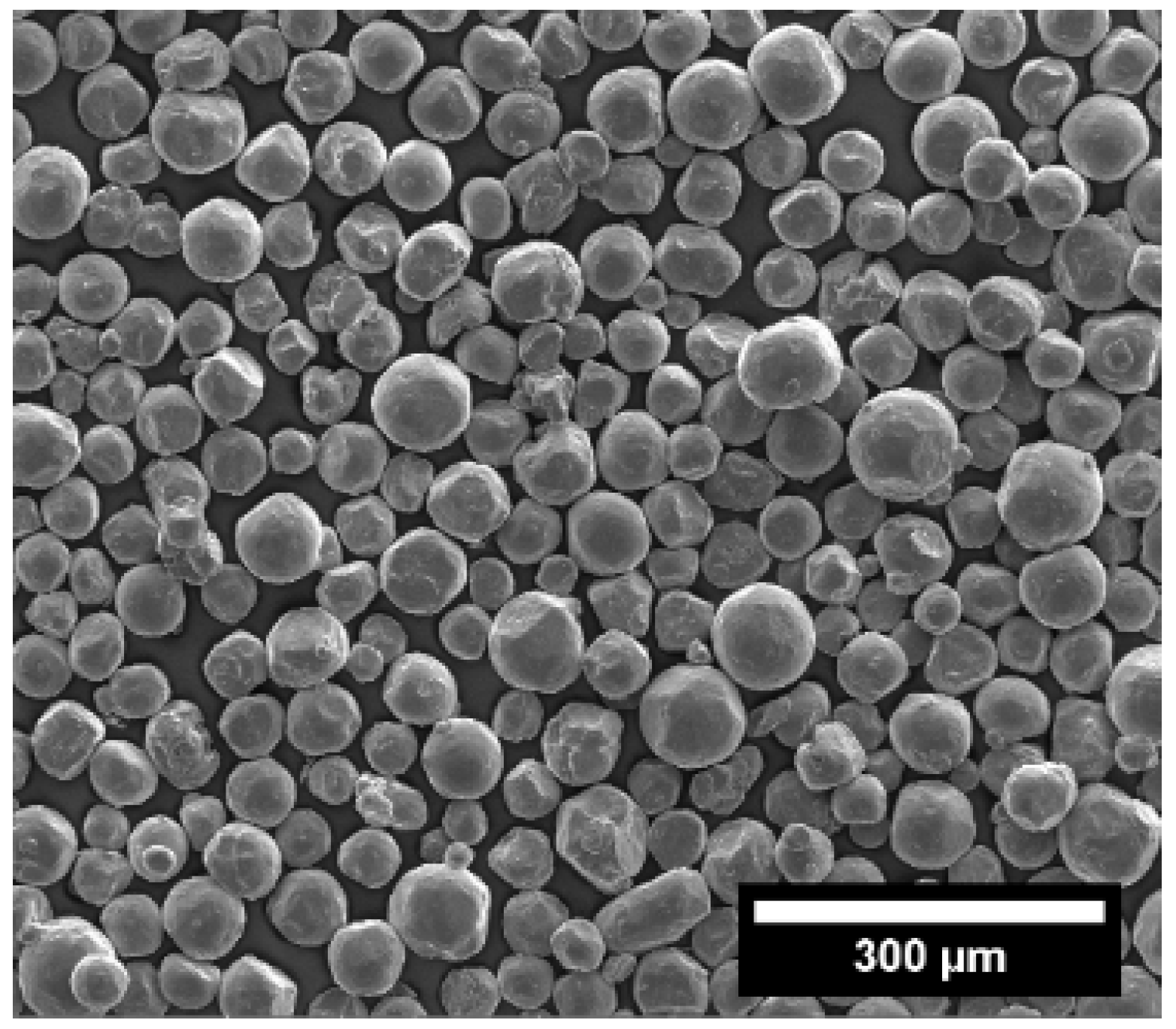
Thoughts on improving grinding efficiency of mill
Factors that affect grinding efficiency include multiple aspects, such as whether the process design, layout, equipment selection, raw materials, process parameter selection, etc. are reasonable, whether the personnel training and operation level, system management are in place, etc. Generally speaking, the process design, layout, and equipment selection are fixed after the factory is built and are difficult to change. To achieve or even exceed the design goals, it depends on the management, operation control, and technical transformation. Such as raw material management; process parameter selection; mill structure adjustment; and the quality of operators, stability of control, etc.
1. Changes and responses to materials entering the mill
1.1 Particle size of materials entering the mill
The company's cement grinding system is a modified open-circuit mill with a pre-mill roller press. Due to the extrusion and crushing of the pre-mill roller press, and then the dispersion and classification, the particle size and grindability of the materials entering the mill have been greatly improved. The original particle size of the materials entering the mill was 20-40 mm, and after the transformation, most of the materials entering the mill were powder.
1.2 Grindability of materials entering the mill
Among the materials entering the mill, the most difficult to grind are clinkers. The clinker has a dense structure, good crystallization, and is not easy to grind.
1.3 Moisture content of materials entering the mill
Combined with expert analysis and multiple tests, our experience is that the comprehensive moisture content of materials entering the mill is controlled at about 2.0%.
1.4 Temperature of materials entering the mill
The temperature of materials entering the mill also has a great influence on the output of the mill and the quality of cement. Appropriate temperature of materials entering the mill plays a good drying role, and can also effectively control the temperature in the mill to ensure good grinding conditions and avoid "ball wrapping" and gypsum dehydration.
2. Adjustment of steel balls and steel forgings
Steel balls and steel forgings are still common in cement production as grinding media. In addition to material requirements, gradation and filling rate are two important indicators. Whether they are reasonable or not not only directly affects the quality of cement production, but also affects the power consumption of cement, which directly leads to changes in costs. With the implementation of new cement standards in my country and the improvement of concrete construction requirements, higher requirements are placed on cement fineness and particle gradation, and thus higher requirements are placed on cement grinding systems. Therefore, in cement production management, these two issues should be paid attention to.
3. Adjustment of mill structure
Cement mills are generally divided into 2 to 3 chambers. According to the company's situation, after adding the pre-mill roller pressing system, the particle size of the mill is greatly reduced, the crushing and coarse grinding functions of the first chamber are weakened, and the length of the second and third chambers is increased to enhance the grinding capacity. At the same time, the lining plate, the partition plate form, and the size of the grate hole are also adjusted accordingly, and a screening device is added inside the mill, which has a good effect. In addition, the mill bearing is changed from a sliding bearing to a rolling bearing, which reduces the starting current and working current, reduces the maintenance amount, and improves the operation rate. Due to the reduction in power usage, a certain amount of steel ball and steel forging load can be added, so the motor efficiency is improved, the useless work is reduced, and the hourly output can be increased, which improves the operation effect of the mill.
High-value application of silicon micro powder
![]()
Silicon micropowder is a non-toxic, odorless, pollution-free inorganic non-metallic material, made from natural quartz (SiO2) or fused quartz (amorphous SiO2 after natural quartz is melted at high temperature and cooled) through multiple processes such as crushing, ball milling (or vibration, air flow milling), flotation, acid washing and purification, and high-purity water treatment.
1 Application in copper-clad laminates
Silicon micropowder is a functional filler. When added to copper-clad laminates, it can improve the insulation, thermal conductivity, thermal stability, acid and alkali resistance (except HF), wear resistance, flame retardancy, bending strength and dimensional stability of the laminates, reduce the thermal expansion rate of the laminates, and improve the dielectric constant of the copper-clad laminates. At the same time, due to the abundant raw materials and low prices of silicon micropowder, it can reduce the cost of copper-clad laminates, so its application in the copper-clad laminate industry is becoming more and more extensive.
Ultrafine crystalline silicon powder
The average particle size of ultrafine silicon powder currently used in copper-clad laminates is 1-10 microns. As the substrates of electronic products develop towards ultra-thinness, fillers are required to have smaller particle sizes. In the future, copper-clad laminates will use ultrafine fillers with an average particle size of about 0.5-1 microns.
Fused silicon powder
Fused silicon powder is a powder made of natural quartz, which is melted at high temperature and cooled with amorphous silicon dioxide as the main raw material, and then processed by a unique process. Its molecular structure arrangement changes from ordered arrangement to disordered arrangement. Due to its high purity, it presents stable chemical properties such as extremely low linear expansion coefficient, good electromagnetic radiation, and chemical corrosion resistance, and is often used in the production of high-frequency copper-clad laminates.
Composite silicon micropowder
Composite silicon micropowder is a glass-phase silicon dioxide powder material made from natural quartz and other inorganic non-metallic minerals (such as calcium oxide, boron oxide, magnesium oxide, etc.) through compounding, melting, cooling, crushing, grinding, grading and other processes. The Mohs hardness of composite silicon micropowder is about 5, which is significantly lower than that of pure silicon micropowder.
Spherical silicon micropowder
Spherical silicon micropowder is a spherical silicon micropowder material with uniform particles, no sharp corners, small specific surface area, good fluidity, low stress and small bulk density, which is made of selected irregular angular silicon micropowder as raw material and processed by high temperature near melting and near spherical method.
Active silicon micropowder
Using active treated silicon micropowder as filler can significantly improve the compatibility of silicon micropowder and resin system, and further improve the moisture and heat resistance and reliability of copper clad board. At present, the domestic active silicon micropowder products are not ideal because they are only simply mixed with silicon coupling agents. The powder is easy to agglomerate when mixed with resin. Many foreign patents have proposed active treatment of silicon micropowder.
2 Application in high-end epoxy resin potting materials
Epoxy resin potting materials are widely used in the potting process of electronic device manufacturing. Potting is an operation process that uses potting materials to reasonably arrange, assemble, bond, connect, seal and protect the various parts of the electrical device according to the specified requirements. Its function is to strengthen the integrity of electronic devices, improve their resistance to external impact and vibration, improve the insulation between internal components and circuits of electronic devices, avoid direct exposure of internal components and circuits of electronic devices, and improve the waterproof, dustproof and moisture-proof performance of electronic devices.
3 Application in epoxy molding compound
Epoxy molding compound (EMC), also known as epoxy resin molding compound or epoxy molding compound, is a powder molding compound made of epoxy resin as the base resin, high-performance phenolic resin as the curing agent, silicon micropowder and other fillers, and a variety of additives. 97% of the global integrated circuit (IC) packaging materials use epoxy molding compound (EMC). The molding process is to extrude EMC into a special mold cavity by transfer molding, embed the semiconductor chip in it, and complete cross-linking and curing molding to form a semiconductor device with a certain structural appearance. In the composition of EMC, silicon micropowder is the most used filler, accounting for 70% to 90% of the weight of epoxy molding compound.
Quality requirements for quartz sand for various types of glass

Silicon dioxide is the main structure of glass, which can ensure that the glass has high strength and good chemical stability. Therefore, quartz sand is the most important industrial mineral raw material in the glass industry, including flat glass, daily glass, ultra-white glass, photovoltaic glass, quartz glass, etc.
The quality requirements of quartz sand in the glass industry are mainly reflected in three aspects: chemical composition, stability, and particle size. Different glass products have different quality requirements for quartz sand.
1. Flat glass
Different flat glass downstream markets have different requirements for quartz sand indicators. According to the chemical composition and particle size, the quartz sand used in the entire flat glass industry can be divided into two types: Class I and Class II. Class I has a low Al2O3 content, and Class II has a high Al2O3 content.
2. Daily glass
Daily glass products mainly include bottle glass, utensil glass, instrument glass and pharmaceutical glass, which provide various packaging and meet social consumption needs for industries such as food, brewing, beverages and medicine. Quartz sand is the raw material with the largest amount of daily glass batches. The melting temperature of quartz sand is as high as about 1730℃, and the particle size of quartz has the greatest impact on the formation of glass.
In actual production, quartz particles should be angular in shape, with a large surface area, and the batch is not easy to stratify. The particle size range is 60-140 mesh.
3. Ultra-white glass
Ultra-white glass is a new material glass with extremely high light transmittance (light transmittance ≥ 91.5%), iron impurity content basically controlled between 100~150ppm and extremely transparent appearance. Other names for ultra-white glass are low-iron glass and high-transparency glass.
The raw materials for ultra-white glass production mainly include quartz sand, feldspar, dolomite, limestone, heavy alkali, aluminum hydroxide, sodium sulfate, sodium pyroantimonate and antimony trioxide, etc., and the requirements for the percentage of various raw materials are very strict. In order to meet the use requirements of ultra-white glass, the industry has strict regulations on the composition of ultra-white glass.
4. Photovoltaic glass
Photovoltaic glass is mainly installed on the outermost layer of photovoltaic modules to block the influence of moisture and corrosive gases, and protect the cells and electrodes. Compared with ordinary glass, photovoltaic glass needs to have low iron content, high light transmittance, impact resistance, corrosion resistance, high temperature resistance and other characteristics. Ultra-white float glass and ultra-white rolled glass can meet the above requirements. Among them, ultra-white rolled glass is used for crystalline silicon cells and is the mainstream product of photovoltaic glass, while ultra-white float glass is mostly used for thin-film cells.
The iron ions in quartz sand are easy to dye. In order to ensure the high solar transmittance of the original glass, the iron content of photovoltaic glass is required to be lower than that of ordinary glass. Low-iron quartz sand with high silicon purity and low impurity content must be used.
5. Quartz glass
Quartz glass is known as the "crown" of glass materials. It is a glass with SiO2 as a single component and has superb mechanical, thermal, optical and electrical properties. It plays an irreplaceable role in semiconductors, optical devices, optical communications, solar energy and other industries. High-purity quartz sand is currently the main raw material for replacing crystal ore and melting quartz glass. Quartz glass produced by electric melting process and gas refining process uses high-purity quartz sand as raw material.
Introduction of Pigment Powder Ultrafine Grinding Equipment

Particle size is one of the important indicators of pigments. Generally, it is required that the pigment particles have a stable physical form, uniform particle size, and good dispersibility without agglomeration or precipitation.
At present, common ultrafine grinding equipment includes air flow mill, mechanical impact ultrafine grinder, stirring ball mill, sand mill, vibration mill, colloid mill, high-pressure jet grinder, planetary ball mill, roller mill, ring roller mill, etc.
1. Air flow mill
Air flow mill is one of the most important ultrafine grinding equipment, and the product fineness can generally reach 1-45μm.
Working principle:
Use high-pressure air, inert gas or superheated steam to expand and cool down to form a high-speed flow field, drive the material particles to collide, rub and shear with each other in the jet flow field to achieve material refinement. Common types include flat type, fluidized bed reverse jet type, circulating tube type, opposite spray type, target type and dozens of specifications.
2. Mechanical impact ultrafine pulverizer
Mechanical impact ultrafine pulverizer is the ultrafine pulverizing equipment that is widely used in the domestic non-metallic mineral industry. The product fineness can generally reach d97=10μm, that is, the so-called 1250 mesh. It can produce ultrafine powder products with d97=5-7μm after being equipped with a high-performance fine classifier.
Working principle:
Using a rotating body (rod, hammer, blade, etc.) rotating at high speed around a horizontal or vertical axis, the feed is violently impacted, causing it to impact and collide with a fixed body or particles, and the ultrafine grinding equipment that crushes the particles with a stronger force has two crushing effects, impact and friction, and also has air flow crushing.
3. Stirring ball mill
A stirring ball mill is a type of ultrafine grinding equipment consisting of a stationary cylinder filled with grinding media and a rotating stirrer. The fineness of the product can reach less than 1μm.
Working principle:
The stirring medium is stirred by the stirrer to produce irregular motion, and the material is subjected to impact or shock, shearing, friction and other effects to crush the material, including intermittent stirring mill, continuous stirring mill, spiral stirring mill, tower mill, grinding and flaking machine, etc.
4. Sand mill
Sand mill is another form of stirred mill, named because it originally used natural sand and glass beads as grinding media. It can be divided into open type and closed type, each of which can be divided into vertical and horizontal types.
Working principle:
The slurry that has been stirred and mixed in the slurry barrel at high speed is pumped into the closed grinding chamber by pumping, and contacts with the high-speed rotating grinding media, so that the solid particles in the material and the grinding media produce stronger collision, friction and shearing effects with each other, so as to accelerate the grinding of particles and disperse aggregates.
Vibration mill is a fine grinding and ultrafine grinding equipment that uses grinding media (spherical or rod-shaped) to impact, rub, shear and other effects on materials in a high-frequency vibrating cylinder to crush the materials. It can process ultrafine powder products with an average particle size of 1μm or even less than 1μm. For materials with greater brittleness, submicron products can be obtained relatively easily.
6. Colloid mill
Colloid mill is a new type of equipment for wet ultrafine particle processing, suitable for various types of emulsification, dispersion, crushing and grinding. The particle size of the processed product can reach several microns to less than 1 micron.
7. High-pressure jet crusher
This type of equipment uses the strong impact force of the high-pressure jet and the cavitation effect after the pressure is suddenly reduced to crush the material due to impact and explosion. The average particle size of the product can be adjusted within the range of 1-20μm.
8. Ring roller mill, pressure roller mill
Ring roller mill and pressure roller mill both use material layer extrusion and crushing technology to achieve ultra-fine crushing of materials. That is, the material produces stress concentration under high pressure, causing cracks and expansion, and then produces numerous micro cracks, forming surface cracks and finally achieving material crushing.
Five reasons that may cause low grinding efficiency of ball mill

The grinding efficiency of ball mill is affected by many factors, including: the movement of steel balls in the barrel, the rotation rate, the addition and size of steel balls, the material level, and the use of grinding aids. These factors have an impact on the efficiency of ball mill to a certain extent.
1. Movement pattern of steel balls in the barrel
To be precise, to a certain extent, the movement pattern of the grinding media in the barrel affects the grinding efficiency of the ball mill.
The working environment of the ball mill is divided into the following categories:
(1) In the surrounding and falling motion areas, the filling amount in the barrel is small or even non-existent, so that the material can make uniform circular motion or falling motion in the barrel, and the probability of collision between steel balls increases, causing wear between the steel balls and the liner, further reducing the efficiency of the ball mill;
(2) In the falling motion area, the filling amount is appropriate. At this time, the steel balls have an impact on the material, making the ball mill efficiency relatively high;
(3) In the area around the center of the ball mill, the steel balls have a circular motion or a mixture of falling motion and falling motion, which limits the range of motion of the steel balls and reduces wear and impact;
(4) In the blank area, the steel balls do not move. If the filling amount is too large, the range of motion of the steel balls is small or does not move, which will cause waste of resources and easily cause the ball mill to malfunction.
2. Rotation rate
An important working parameter of the ball mill is the rotation rate, which directly affects the grinding efficiency of the ball mill. When considering the rotation rate, the filling rate should also be considered. The filling rate is positively correlated with the rotation rate. When discussing the rotation rate here, keep the filling rate constant. No matter what the motion state of the ball load is, there will be an optimal rotation rate at a certain filling rate.
When the filling rate is constant and the rotation rate is low, the energy obtained by the steel ball is low, and the impact energy on the material is low. It may be lower than the threshold of the ore particle crushing, resulting in ineffective impact on the ore particles, that is, the ore particles will not be crushed, so the grinding efficiency at low speed is low.
3. Addition and size of steel balls
If the amount of steel balls added is inappropriate, the ball diameter and ratio are unreasonable, then the grinding efficiency will be reduced. The ball mill is subject to greater wear during operation, and a large part of the reason is that the manual addition of steel balls is not well controlled, resulting in the accumulation of steel balls and the phenomenon of ball jamming, which in turn causes certain wear on the machine.
4. Material level
The material level affects the filling rate, which in turn affects the grinding effect of the ball mill. If the material level is too high, it will cause coal blockage in the ball mill. Therefore, effective monitoring of the material level is very important. At the same time, the energy consumption of the ball mill is also related to the material level. For the intermediate storage type powder making system, the power consumption of the ball mill accounts for about 70% of the power consumption of the powder making system and about 15% of the power consumption of the plant. There are many factors that affect the intermediate storage type powder making system, but under the influence of many factors, effective inspection of the material level is very necessary.
5. Liner selection
The liner of the ball mill can not only reduce the damage to the cylinder, but also transfer energy to the grinding medium. One of the factors affecting the grinding efficiency of the ball mill is determined by the working surface of the liner. In practice, it is known that in order to reduce the damage to the cylinder and improve the grinding efficiency, it is necessary to reduce the sliding between the grinding medium and the liner. Therefore, the main method is to change the shape of the working surface of the liner and increase the friction coefficient between the liner and the grinding medium. High manganese steel liners were used before, and now there are rubber liners, magnetic liners, angle spiral liners, etc. These modified liners are not only higher in performance than high manganese steel liners, but also can effectively extend the service life of the ball mill.
Targeted improvements in the movement of the ball mill's steel balls, rotation speed, addition and size of the steel balls, material level, and liner material can effectively improve grinding efficiency.
Why does quartz sand need modification?

The reasons why quartz sand needs modification mainly include the following aspects:
change surface properties
Surface modification of quartz sand can change its physical and chemical properties such as lipophilicity, wettability, oil absorption rate and viscosity. These changes help improve the performance of quartz sand in a variety of applications.
Improve compatibility with organic polymers
When quartz sand is used as a filler, it is very important to improve its compatibility, affinity, dispersion and fluidity with organic polymers. Through surface modification, these properties can be significantly improved, allowing the quartz sand to better mix and combine with materials such as resin.
Enhance adsorption performance
Surface modification of quartz sand can also improve its adsorption performance for heavy metal ions. For example, by modifying it with metal salts such as aluminum chloride and magnesium chloride, the adsorption effect of quartz sand on heavy metal ions can be significantly improved.
Expand application areas
Surface modification is an effective way to open up new application fields of quartz sand. Through modification, modified filter materials with excellent adsorption performance and certain mechanical strength can be made, which are widely used in water treatment, air purification and other fields.
Increase industrial value and added value
Surface modification of quartz sand not only optimizes its properties, but also increases its industrial value and added value. This is of great significance for achieving efficient utilization and economic benefits of quartz sand.
Addressing practicality limitations
Due to the smooth surface of quartz sand and limited active sites, it is easy to cause rapid saturation of the adsorption sites, affecting its practical application effect. Through surface modification, the active sites on the surface can be increased, thereby improving its practicality in filter media and other aspects.
Quartz sand needs to be modified in order to optimize its physical and chemical properties, improve its compatibility with other materials, enhance adsorption performance, expand its application areas, and enhance its industrial value and added value, so as to better meet the needs of modern industry for high performance Material requirements.
Which high-end powders require surface modification?
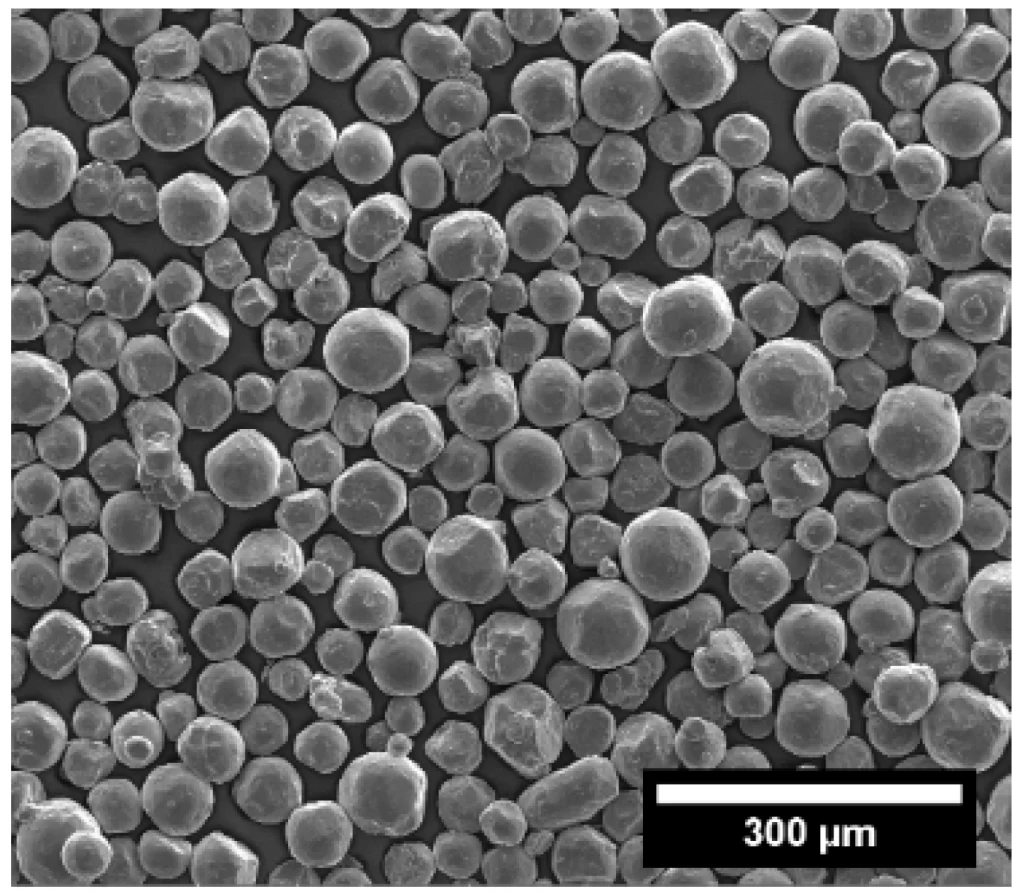
High-end powders that require surface modification mainly include inorganic powders and ultrafine powders. Here are specific examples and why:
Inorganic powder
Inorganic powders such as porous silica, silica powder, etc. can increase their surface hydroxyl content through surface modification and enhance the hydration effect, thus improving their compatibility and mechanical properties in composite materials. In addition, surface modification of inorganic powders can also improve their gloss, tinting power, hiding power, color retention and weather resistance.
Ultrafine powder
Since ultrafine powder has a small particle size and high surface energy and is prone to agglomeration, surface modification is required to prevent agglomeration and impart new functionality, such as hydrophilicity or lipophilicity. For example, in the cosmetics industry, surface modification of powders must not only block their catalytic activity, but also impart the required functionality.
Metal powder
Surface modification technology of metal powders can be used to extend the service life of parts and improve performance, making it possible to prepare metal powder materials with better performance.
Surface modification of these powders usually involves physical, chemical or mechanical methods to change the physical and chemical properties of the powder material surface to meet the needs of specific applications. For example, the surface of porous silica can be activated by microwave radiation and air plasma treatment, or the modifier can be evenly distributed on the outer surface of the powder particles using mechanical forces such as extrusion, impact, shearing, and friction.
In summary, high-end powders that require surface modification mainly include inorganic powders, ultrafine powders and metal powders. The purpose of modification is mainly to improve the performance of the powder, increase functionality and improve the compatibility with other substances. Capacity.
What are the applications of natural cellulose powder and protein powder?

There is a waste problem of natural cellulose and natural protein in the textile industry, agriculture, papermaking and other fields. Natural cellulose and natural protein that have been processed many times cannot degrade by themselves and will cause serious environmental pollution. Therefore, how to efficiently recycle and utilize them Waste natural fiber materials have become a research hotspot. Usually when a material is processed into powder, its properties will undergo a series of changes, such as specific surface area, surface energy, surface activity, surface and interface properties, and crystallinity.
Application of natural cellulose powder
(1) Medical applications
As a new biomedical material, cellulose powder not only acts as a natural barrier to prevent allergens from combining with the nasal mucosa, but can also reduce allergic symptoms in children who are sensitive to insects; because it is a natural cellulose powder, it can be used by pregnant women and special groups. use.
(2) Applications in food and packaging materials
Most of the current food packaging materials are non-degradable, and degradable food packaging bags can greatly alleviate environmental pollution problems. Cellulose is non-toxic and a renewable resource. It is a good material for making degradable food packaging bags.
(3) Application of flame retardant materials
Using natural cellulose powder to replace the carbon source pentaerythritol in the traditional intumescent flame retardant system not only changes the shortcomings of the large amount of carbon source and poor compatibility in the traditional intumescent flame retardant system, but also increases the number of intumescent carbon layers and reduces the flame retardancy.
(4) Applications in sensing materials
Nano-zinc oxide (ZnO) ultraviolet (UV) sensors can be produced using a simple and low-cost two-step chemical method, so they have attracted much attention from researchers. Studies have found that the UV sensing activity of nano-ZnO can be significantly enhanced by synthesis with cellulose polymers.
Application of natural protein powder
(1) Applications in biomedical materials
Protein powder is widely used in biomedical materials due to its good biodegradation and biocompatibility. Using silk fibroin powder and polyacrylamide to make new hydrogels can improve the mechanical properties of the hydrogel, making it adhesive and self-healing. It has broad application prospects in wound dressings and transparent artificial skin. Protein powder also has great application potential in the development of small-diameter textile-based artificial blood vessels.
(2) Applications in composite materials
Mixing natural protein powder with other polymer materials to prepare new natural polymer materials can improve processing performance, etc., and provides a new direction for manufacturing natural-synthetic composite polymer materials. Natural protein powder, graphene oxide and catalyst nickel are used as raw materials to make conductive composite materials.
(3) Application of additives
Protein powder is added to coatings as a breathable agent and applied to clothing to improve its breathability. The biggest disadvantage of coatings applied to fabrics is poor air permeability. Adding silk fibroin protein powder to protective coatings that prevent thermal radiation improves the permeability of protective clothing to water vapor and air, and provides improvements to fabrics after coating.
Cellulose powder and protein powder with good application prospects are obtained from waste fabrics, agricultural waste and other waste materials, realizing the environmental protection concept of waste recycling. The biodegradability and biocompatibility of cellulose powder and protein powder are Capacitive is also widely used in medicine and materials, but the preparation efficiency of cellulose powder and protein powder is low, and the common preparation method of cellulose requires a large number of chemical reagents, and the degree of reaction is difficult to control; Preparation method of protein powder Traditional drying methods have low yields, and centrifugal separation from solvents is prone to agglomeration. Based on these problems, more efficient and low-energy consumption preparation methods should be innovated according to their own characteristics. With the continuous research on renewable natural protein powder and natural cellulose powder, more new application fields are developed, such as cosmetics and coatings. In the near future, natural protein powder and natural cellulose powder will create greater value.
Application of aluminum nitride in the field of high thermal conductivity

At present, the application of aluminum nitride in the field of high thermal conductivity mainly focuses on two aspects: packaging substrate and thermal conductive filler.
Ideal electronic packaging substrate material
The packaging substrate mainly uses the high thermal conductivity of the material itself to conduct heat away from the chip (heat source) to achieve heat exchange with the external environment. For power semiconductor devices, the packaging substrate must meet the following requirements:
(1) High thermal conductivity;
(2) Match the thermal expansion coefficient of the chip material;
(3) It has good heat resistance, meets the high temperature use requirements of power devices, and has good thermal stability;
(4) Good insulation, meeting the electrical interconnection and insulation requirements of the device;
(5) High mechanical strength, meeting the strength requirements of device processing, packaging and application processes;
(6) The price is appropriate and suitable for large-scale production and application.
Thermal conductive filler
With the miniaturization and high integration of electronic products and their devices, heat dissipation issues have become an important bottleneck restricting the development of electronic technology, and thermally conductive composite materials such as thermal interface materials, which determine the heat dissipation effect, have attracted more and more attention.
Currently, commercial thermally conductive composite materials are generally composed of polymers and thermally conductive fillers. Since the thermal conductivity of polymers is very low, generally less than 0.5W/m·K, the thermal conductivity of thermally conductive composite materials is mainly determined by thermally conductive fillers. At present, the most widely used fillers on the market are oxide fillers represented by Al2O3, etc. However, the intrinsic thermal conductivity of alumina is only 38~42W/m·K. Due to its limitation, it will be difficult to prepare heat dissipation materials that meet the requirements of the future. Thermal conductive composite materials required by the market.
It should be pointed out that although the overall performance of aluminum nitride is far better than that of aluminum oxide, beryllium oxide and silicon carbide, and it is considered an ideal material for highly integrated semiconductor substrates and electronic device packaging, it is prone to hydrolysis by absorbing water in the air. The reaction causes the surface to be coated with an aluminum hydroxide film, which interrupts the thermal conduction path and affects the transmission of phonons. Moreover, its large content of filling will greatly increase the viscosity of the polymer, which is not conducive to molding processing.
In order to overcome the above problems, the surface modification of aluminum nitride thermally conductive particles must be carried out to improve the interface bonding problem between the two. At present, there are two main methods to modify the surface of inorganic particles. One is the surface chemical reaction method, which is the adsorption or reaction of small molecular substances such as coupling agents on the surface of inorganic particles. The other is the surface grafting method, which is a grafting reaction between polymer monomers and hydroxyl groups on the surface of inorganic particles.
Currently commonly used are coupling agent surface modifications, such as silane and titanate coupling agents and other types of surface treatment agents. Compared with the surface chemical reaction method, the surface grafting method has greater flexibility. It can select monomers and grafting reaction processes that meet the conditions according to different characteristic requirements.
Applications of zeolite in various fields

For many years, zeolite has been mainly used for blood purification in the medical field. In developed countries such as Europe and the United States, micronized zeolite has been hailed as a "natural medical device" in the medical field.
Because zeolite itself has a regular porous structure and small particle size, it can filter molecules, exchange cations, and adsorb heavy metal substances. Therefore, after zeolite enters the human body, it can adsorb and remove a variety of toxins, radioactive elements and other harmful metabolites in the human body.
In recent years, natural zeolite has been widely used in green building materials, petrochemical industry, soil improvement, sewage treatment, metallurgy, medicine, atomic energy industry and light industry, becoming an important new natural and environmentally friendly material in the national economy. Therefore, the development of natural zeolite and applications are attracting more and more attention.
1. In the petroleum and chemical industries: used as catalytic cracking, hydrocracking in petroleum refining and chemical alienation, reforming, alkylation and disproportionation of petroleum; gas and liquid purification, separation and storage agents; hard water softening and seawater desalination. Agent; special desiccant (dry air, nitrogen, hydrocarbons, etc.).
2. In the light industry: used in papermaking, synthetic rubber, plastics, resins, paint fillers and quality colors, etc. It is used as adsorption separation agent and desiccant in national defense, space technology, ultra-vacuum technology, energy development, electronic industry, etc.
3. In the field of green building materials: This is the largest application field of zeolite. According to statistics, two-fifths of the world's zeolites are used in the building materials industry, which can effectively improve the performance of concrete; or used in wall decoration materials. Zeolites have strong adsorption capabilities and can absorb polar molecules such as H2O, NH3, H2S, CO2, etc. have high affinity and can still be effectively adsorbed even under conditions of low relative humidity, low concentration, and high temperature. 4. In agriculture: Zeolite can be used as a soil conditioner to maintain moisture, fertility, and adjust pH. In the production of chemical fertilizers and pesticides, zeolite can be used as filler and solidification dispersion medium.
5. In terms of environmental protection: Zeolite can be used to treat waste gas and waste water, remove or recover metal ions from waste water and liquid, and remove radioactive pollutants from waste water.
6. In medicine: Zeolite is used to measure the amount of nitrogen in blood and urine. Zeolite has also been developed as a health product for anti-aging and removing heavy metals accumulated in the body.
7. In supply: Zeolite is often used in the refining of sugar.
8. Raw materials for new wall materials (aerated concrete blocks): As solid clay bricks gradually withdraw from the stage, the application proportion of new wall materials has now reached 80%. Wall material supply companies use coal gangue, fly ash, ceramsite, Slag, light industrial waste, heavy construction waste, zeolite, etc. are used as main materials to actively develop new wall materials.
9. In chemical distillation or heating experiments: often used to prevent bumping. There are a large number of small pores in the structure of zeolite, which can be used as condensation nuclei of bubbles to make the reaction liquid boil smoothly. Bisque-fired porcelain pieces broken to the size of rice grains can be used instead.
10. It can be used as a fish and shrimp feed additive in aquaculture, and can also be used as fish pond construction materials to purify water quality. Ammonia filtration for fish hatcheries; biological filter media.