Development, technology status and future development trend of modified plastics industry

Due to the rapid development of the plastics industry, the filler masterbatch is no longer used as a single filler material. People use more advanced processes from the production methods of open refining and banburying, adding inorganic materials, chemical additives and other materials. Their respective characteristics and commonalities, and then the use of twin-screw extruders and triple-screw extruders for mixing and extrusion has become an important way and method for people to improve the special properties of plastic products. Plastic filling modification is the fastest growing in recent years. A new industry in the plastics industry.
1. Application of 8 major modified plastics downstream market
Automotive industry; Home appliance industry; Electronic and electrical industry; Machinery and equipment industry; Railway/military/medical/aerospace.
2. Five types of plastic modification methods
(1) Modified filling
The main purpose of filling masterbatch is to reduce production cost. Most of them are produced by using inorganic powder or industrial waste with low price and wide sources as filling material, and adding appropriate amount of additives and synthetic resin.
(2) Modified masterbatch
Modified masterbatch is a new modified material developed on the basis of filler masterbatch. Add inorganic materials such as glass fiber, talc, mica, wollastonite, barium sulfate, kaolin to the resin, or add synthetic resins or auxiliaries with special properties during processing, such as: anti-aging agent, antioxidant, anti-aging agent These composite materials play the functional characteristics of different materials in application.
(3) Functional modification
Various materials such as graphene, silicone powder, rare earth, magnesium hydroxide, metal fine powder (silver, copper, zinc, etc.) are added to the plastic, and the product index is improved through modification technology, and the flame retardancy, aging resistance, resistance Physical properties such as high and low temperature have been improved, and special properties such as electrical conductivity, antibacterial, insulation, and reinforcement can also be realized, and it has occupied a place in the main durable plastic product market.
(4) Multi-component compound modification
Multi-component composite modification mainly combines plastics with one or more inorganic materials, polymer materials, chemical additives, etc. through blending, grafting, block and other forms to make plastics "alloying". The properties of each component complement each other to form a plastic material with multiple excellent properties, so as to achieve the purpose of improving performance and multi-functionality.
(5) Special modification
Different functional materials or additives are added to the special plastics, so that the expensive special plastics not only maintain the original characteristics, but also have special functions, which are suitable for the market application of various products.
3. Three new trends in the development of modified plastics
(1) Nanoscale inorganic materials
Inorganic materials are widely used in plastics. The functions of inorganic materials are gradually highlighted with the ultra-fine particle size. Plastics modified with inorganic nano-powders have many unique properties, bringing new development opportunities to the development of the plastics industry.
(2) High-efficiency chemical additives
The development of new high-efficiency additives has become an important development direction for modified plastics. The additives involved in modified plastics are in addition to those commonly used in plastic processing, such as heat stabilizers, plasticizers, UV absorbers, nucleating agents, antistatic agents, In addition to dispersants and flame retardants, high-efficiency and multi-functional functional additives such as toughening, flame retardant, synergistic, and alloy compatibility (interface compatibility) are also critical to modified plastics.
(3) Environmental protection of modified plastics
With the enhancement of people's awareness of environmental protection and the increasingly strict environmental regulations, the concepts of environmental protection such as plastics' renewable utilization, environmental digestibility, biodegradability, non-toxicity, odorless, and pollution-free have been integrated into the design and manufacture of modified plastics In the process, attention should be paid to the conservation and rational utilization of energy resources, and the research and development of non-polluting, fully degradable, recyclable and environmentally friendly modified plastic products has become a new hotspot.
Preparation technology of clay mineral-organic polymer composite bactericidal material
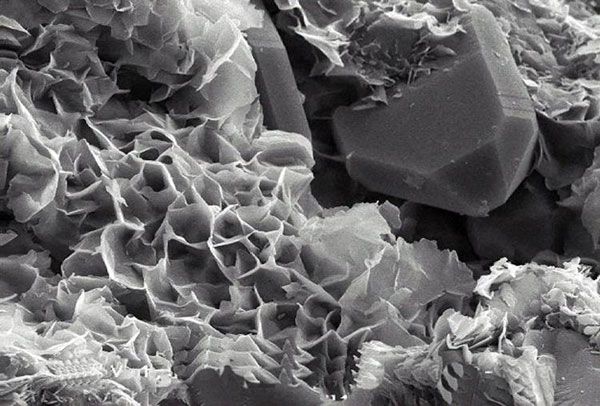
Among the new bactericidal materials based on clay minerals, clay minerals themselves are mainly used as carriers for loading bactericidal substances (such as metals, metal oxides, organic substances), and their bactericidal ability is still limited. A variety of methods are used to prepare modified clay minerals, and the composites made of clay minerals and other materials can be used as new bactericidal materials to produce bactericidal effects on a variety of bacteria.
Clay minerals can enhance the bactericidal ability through various modification methods (including thermal modification, acid modification, inorganic modification of metals or metal oxides, organic modification and composite modification, etc.). The surface area is increased, the mineral porosity and dispersibility are improved, and the overall thermal stability and mechanical strength of the material are improved. The clay minerals used for modification and preparation of bactericidal materials are mainly montmorillonite, kaolinite, halloysite, and vermiculite. is widely used for its adsorption capacity.
The preparation of clay minerals-organic polymer bactericidal materials usually refers to the addition of organically modified clay minerals to the organic polymer matrix to enhance the physicochemical properties and bactericidal activity of the materials. Such materials are mainly used to prepare nanofiber antibacterial cotton cloths, cotton pads and films. etc., clay minerals are used as fillers in composites to enhance the thermal and mechanical stability of nanomaterials, and clay minerals are usually at the nanoscale.
In view of the poor compatibility of clay minerals with organic molecules, organic compounds are often used to modify clay minerals to increase the dispersibility of clay minerals in organic solvents and ensure high compatibility between subsequent organic compounds and modified clay minerals. sex. Organic modification often uses anionic and cationic surfactants (quaternary ammonium salts and hybrid compounds are the most common), by changing the surface properties of the clay (changing the surface electrical properties and the surface hydrophobicity) or inserting organic matter into the interlayer (increasing the interlayer). domains and become hydrophobic between layers) to achieve modification. The organic polymer matrix used mainly includes polypropylene, polyethylene, polyethylene terephthalate, polyurethane, polystyrene, polyamide, polyolefin and the like. Biopolymers such as cellulose, starch, corn-derived plastics, polylactic acid, etc. have received attention due to their environmental friendliness and renewability.
Due to the high compatibility between organomodified clays and polymers, organomodified clays have become ideal materials for enhancing the properties of polymer matrices and are widely used as precursors for bactericidal materials. The properties of composite bactericidal materials are affected by the size scale of different components and the degree of mixing between multiple phases. During the preparation process, three types of intercalated composite materials, clay-exfoliated composite materials and flocculated composite materials are usually formed.
In intercalated nanocomposites, polymer chain moieties are regularly inserted between layers of clay. In exfoliated nanocomposites, the individual clay structural unit layers are relatively uniformly separated in the continuous polymer matrix, and the clay structural unit layers are completely exfoliated in the polymer matrix. The flocculated nanocomposite refers to the phenomenon of “flocculation” similar to the interaction of the hydroxylated edges between the clay mineral structural unit layers, the interlayer domain is reduced, and the polymer and the clay mineral phase are separated to a certain extent.
To study the bactericidal activity of Cu-loaded montmorillonite-chitosan nanocomposites. The synthesis of the composite is accomplished by ion exchange by placing montmorillonite in a medium containing copper sulfate. The lethality rate of the bactericidal material to Escherichia coli is as high as 99.98%, and all Staphylococcus aureus died after being treated with the material.
Application and Market Prospect of Silica in Food and Cosmetics

Silica is a safe and environmentally friendly daily chemical additive, and has better performance in high-end applications. For example, as beer silica gel in food to make products taste better, and as an anti-caking agent in cosmetics, it is harmless to the environment.
Regulatory agencies in various countries have certified silica as a safe and harmless additive. Regulatory agencies in Europe, the United States, and the United Nations have approved silica as an additive for use in food and other fields. A 2006 study by the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC) showed that human inhalation of silica through the mouth, skin or eyes is essentially non-toxic and has no significant impact on environmental quality.
1. Application and substitution of silica in the food field
Silica has the excellent properties of non-toxic, harmless, stable properties and large specific surface area, which is exactly in line with the characteristics of food anticaking agents and adsorbents, and is more high-quality and efficient than the original products.
In the field of table salt, silicon dioxide is not only non-toxic, but also has high resistance to caking, which is superior to potassium ferrocyanide and ferric ammonium citrate, and can be used as a green and healthy anti-caking agent in table salt products. .
In the field of beverages such as beer and fruit juice, silica can agglutinate turbid substances and has a clarifying effect. It can effectively remove turbid proteins in beer without affecting the quality of beer products, and the loss of beer in the whole process is very small. Compared with other filter aids, it has the advantages of less dosage and better effect, and has been widely used in the beer industry as a new type of environmentally friendly adsorbent.
In the field of edible oil, adding less silica can greatly reduce the amount of activated clay used, avoid the color of the edible oil being too light, not only can get better quality sunflower oil, but also help enterprises save production costs.
2. Application and substitution of silica in the field of cosmetics
Plastic microbeads have been banned from production due to environmental issues, and silica is widely used as an excellent ingredient in cosmetics. Silica is a GRAS (Generally Recognized as Safe) ingredient in personal care products such as cosmetics and sunscreens, and its spherical, small particle size, and porous properties make it an anti-caking agent in the cosmetic field And thickener, can improve storage stability and dispersion of powder products, is widely used, such as dispensing lipstick and cosmetic pigments to help improve the free-flow properties of hair bleach and nail polish coating properties.
3. The growth of high-end daily chemical applications of silica is exploding
Silicone rubber is odorless and non-toxic, suitable for a wide range of working temperatures, and has good insulation, oxidation resistance, light resistance, mildew resistance, and chemical stability. With the improvement of people's requirements for quality of life, it is widely used in daily chemical consumption such as baby care products. middle.
The fields of food and cosmetics are relatively scattered, and the potential space for consumption upgrade is huge. The global demand for silica used in food and cosmetics can reach 100,000 tons.
The trend of high-end beer in food is in the ascendant, the price of products is constantly rising, and consumers' demands for product quality and taste are also increasing accordingly. According to the data of Japan's Kirin Holdings (Kirin), the global beer production has reached 191.06 billion liters in 2018. According to beer silica gel The addition of 0.03%-0.06% is assumed, and the global demand is 60,000-120,000 tons.
6 Types Of Flame Retardants Commonly Used In Polypropylene

As one of the five general-purpose plastics, polypropylene (PP) is widely used in all walks of life. However, the flammable characteristics of PP also limit its application space and hinder the further development of PP materials. Therefore, the flame retardancy of PP Modification has always been the focus of attention.
Flame retardant is a booster for polymer synthetic materials. The use of flame retardants can be used to flame retardant polymer materials, so as to avoid material combustion and prevent the spread of fire, and promote the synthetic materials to have smoke suppression, self-extinguishing and flame retardancy. At present, the commonly used flame retardants for polypropylene mainly include metal hydroxide flame retardants, boron-based flame retardants, silicon-based flame retardants, phosphorus-based flame retardants, nitrogen-based flame retardants, and intumescent flame retardants.
1. Metal hydroxide flame retardant
The activated carbon in the metal hydroxide flame retardant has a large specific surface area and is rich in functional groups, which can be well combined with the hydroxyl groups on the sodium magnesium hydroxide particles, effectively weakening the surface polarity of magnesium hydroxide and reducing its occurrence. The possibility of agglomeration improves the compatibility of sodium magnesium hydroxide with the PP matrix, so that the flame retardant properties of the material are enhanced.
2. Boron flame retardant
In the PP/BN@MGO composite, due to the coating structure and alkylation modification of the BN@MGO flame retardant, its alkyl chain grafting efficiency is high, and carbon elements can be enriched on the surface of the filler, which significantly enhances the The affinity between the BN@MGO flame retardant and the PP body enables it to be uniformly distributed in the PP matrix.
3. Silicon flame retardant
HNTs-Si in silicon-based flame retardants can maintain the original tubular structure in the high temperature range, and can twist with the thermally degraded PP chain to form a "fibrous" dense carbon layer, which effectively inhibits the burning of PP. Heat, mass and smoke transfer.
4. Phosphorus flame retardant
In phosphorus-based flame retardants, sorbitol has a large number of hydroxyl groups, which is easy to form a carbonized layer during combustion, while ammonium polyphosphate decomposes when heated to produce phosphoric acid compounds, which further enhances the carbonization of sorbitol, and the generation of carbon layer is delayed. The spread of heat, and the isolation of oxygen, improves the flame retardant properties of the material.
5. Nitrogen flame retardant
MPP will produce incombustible gases (including NH3, NO and H2O) and some phosphorus-containing substances during combustion, while AP can release aluminum phosphate Al2 (HPO4) 3 and phosphine (PH3) gases at high temperatures, these gases not only It can dilute flammable gases in the air, and can also act as a gas shield on the surface of the material, thereby reducing combustion.
6. Intumescent flame retardant
NiCo2O4 has the advantages of controllable morphology, large specific surface area, many active sites, and simple and diverse preparation methods. As a nickel-based compound, NiCo2O4 has excellent carbon catalytic ability, which not only reduces combustion products and improves flame retardancy.
Effect of Silicon Micropowder Content on Properties of Epoxy Castables for Electrical Insulation
Epoxy insulating castable is a liquid or viscous polymerizable resin mixture mixed with resin, curing agent, filler, etc. At the pouring temperature, the castable has the characteristics of good fluidity, less volatile matter, fast curing, and small shrinkage after curing, fixing and isolation and other functions in one insulation product.
Silicon micropowder is one of the important components of insulating castables, and has an irreplaceable role in reducing shrinkage, reducing costs, and improving performance.
At present, insulator manufacturers try to increase the proportion of filler content as much as possible in order to reduce costs. Insulators with too high filler content will greatly reduce their insulation performance, mechanical properties and service life, which will seriously affect the safe and reliable operation of the power system; insulating parts with too low filler content will also reduce their overall performance. Epoxy resin manufacturers have not made reasonable regulations on the addition ratio of fillers, which has brought great confusion to epoxy insulation manufacturers.
Using liquid bisphenol A epoxy resin as the base material, methyltetrahydrophthalic anhydride as the curing agent, BDMA as the accelerator, 400-mesh active silicon powder as the filler, according to different filler ratios, the APG process was used to prepare the test strips. The effects of different amounts of silicon micropowder on the mechanical strength, dielectric properties, solution corrosion resistance and water absorption of epoxy castables were investigated. The results show that:
(1) With the increase of filler content in the epoxy resin system, the dielectric constant and dielectric loss of the sample block generally tend to increase.
(2) When the filler content is low, with the increase of the proportion, the resistance to leakage traces increases. When the filler content reaches 69.42%, the resistance to leakage traces reaches the maximum; after that, with the further increase of fillers, the resistance to leakage traces increases. It started to get worse again.
(3) When the filler content increases to 67.26%, the lye corrosion resistance begins to decline significantly.
(4) The mechanical properties of the samples initially increased with the increase of filler content, and when the filler content increased to 69.42%, the mechanical properties began to fluctuate.
(5) Although the filler content increases, it can reduce the shrinkage rate of the casting, improve its thermal conductivity and rigidity, improve its crack resistance, and reduce production costs, but too high filler content will not only make the process worse, but also It will reduce the insulation performance, mechanical stability and corrosion resistance of the product. Therefore, considering the comprehensive performance, the optimum content range of silicon micropowder is 63% to 67%.
Application and research progress of hydroxide flame retardant in polyethylene

Polyethylene (PE) is a thermoplastic resin obtained by the polymerization of monomer ethylene. It has good cold resistance, good mechanical strength and dielectric properties. It is widely used in cables, films, pipes, packaging, containers, medical appliances and other products. But the PE oxygen index is 17.4%, which is a flammable material. PE material has a fast burning speed, a large amount of heat/smoke, and it is easy to melt and drop when burning, which poses a great threat to the safety of life and property, and limits the use and development of polyethylene. Therefore, it is imperative to carry out flame retardant modification.
Metal hydroxide flame retardants are mainly aluminum hydroxide and magnesium hydroxide. Magnesium-aluminum flame retardants have good stability, non-toxicity, and low smoke generation. During the combustion process, water vapor will be released to dilute the combustible gas, take away part of the heat, inhibit combustion, and produce a flame retardant effect. Aluminum-magnesium flame retardant can prolong the ignition time and reduce the heat release rate. The compatibility of magnesium hydroxide with PE is poor and the flame retardant efficiency is low. It needs a large amount of addition to improve the flame retardant performance, and a large amount of addition will reduce the processing of composite materials. sex and mechanical properties.
Magnesium hydroxide was surface-modified with sodium stearate and polyethylene glycol as modifiers, and high-density polyethylene flame retardant composites were prepared. The research shows that when the addition amount of modified magnesium hydroxide is 30%, the tensile strength of HDPE/magnesium hydroxide composite material is 12.3MPa, magnesium hydroxide has good compatibility with HDPE, and the limiting oxygen index is increased to 24.6% , the flame retardant performance improved less.
Layered double hydroxide will release CO2 and H2O when it decomposes, dilute and block oxygen, making it have a good flame retardant effect and can replace halogen and phosphorus-containing flame retardants.
Aluminum hydroxide/Mg-Fe-LDH/HDPE flame retardant composites were prepared with aluminum hydroxide and self-made magnesium iron double hydroxide (Mg-FeLDH) as flame retardants. The study found that aluminum hydroxide and Mg-Fe-LDH can effectively inhibit the CO release and heat release during the combustion of composite materials (HDPE1, HDPE2, HDPE3), making HDPE difficult to ignite. When the total amount of flame retardants is 40% (2% of Mg-Fe-LDH, HDPE2), HDPE composites have good flame retardant properties.
HDPE composites were prepared with aluminum hydroxide, expanded vermiculite and antimony trioxide as flame retardants. The study found that when the ratio of aluminum hydroxide/expanded vermiculite was 3:2, the mechanical properties of the composite material were better, and the smoke suppression and flame retardant performance reached FV-0 level. When the total amount of aluminum hydroxide and expanded vermiculite is 50%, the limiting oxygen index first increases and then decreases with the increase of aluminum hydroxide, and the optimum ratio is 3∶2.
The effects of magnesium hydroxide and zinc borate on the flame retardant properties of linear low density polyethylene and ethylene ethyl acrylate copolymer were studied. It was found that with the increase of the ratio of magnesium hydroxide and zinc borate, the flame retardant performance of the composite material improved. When the addition amount of magnesium hydroxide was 65%, the flame retardant performance was the best, reaching the UL94V-0 level.
The effect of magnesium hydroxide on the flame retardant properties of linear low density polyethylene was studied. When the dosage of magnesium hydroxide reaches 70%, the limiting oxygen index reaches 31.4%, which is about 71% higher than that of pure material, and the vertical combustion test reaches V-0 level.
Metal hydroxide flame retardants are safe, environmentally friendly and inexpensive. When used alone, the flame retardant effect is not good, and a large amount of addition is required to improve the flame retardant performance of the material, but when a large amount is added, the mechanical properties will be reduced. Therefore, it is the research direction of hydroxide flame retardant to study surface modification and use it in combination with nitrogen and phosphorus flame retardants to improve the flame retardant performance and reduce the addition amount.
High-purity, low-radioactive spherical silica powder production technology

Spherical silicon powder is widely used in integrated circuit packaging due to its excellent fluidity and low thermal expansion coefficient. With the development of large-scale and ultra-large-scale integrated circuit packaging technology, in order to avoid soft errors in semiconductor devices, radioactive elements are obtained. In particular, the high-purity and low-radioactive spherical silica micropowder with uranium (U) content (mass fraction) less than 1×10-9 has become a research hotspot in recent years.
First, the uranium (U6+) element in the ultrafine silicon powder is dispersed in the acidic slurry, and then the SiO2 aerogel and honeycomb ceramic composite mesoporous adsorption device is used to adsorb it to complete the material selection and purification, so that the ultrafine silicon powder The total content of uranium (U) element is reduced to below 1×10-9, and high-purity and low-radioactive spherical silica powder is finally obtained by flame melting method and non-polluting post-processing technology. The mesoporous adsorption device can be easily and quickly separated from the slurry after the adsorption is completed, and can realize recycling and large-scale amplification; and the obtained samples have the characteristics of high sphericity and controllable particle size distribution, and at the same time, the application performance such as fluidity performance good.
1. Selection and purification of microsilica
The first step is to pre-treat the uranium (U)-containing silicon micropowder
The second step is to prepare a mesoporous adsorption device
The third step, adsorption and purification
In the experiment, ordinary silicon powder with a uranium (U) content of 9.7×10-9 was selected for purification. If only deionized water was used to purify the ultra-fine silicon powder, the uranium (U) element content in the material was only from 9.7×10-9 It is reduced to 9.0×10-9; when the ultrafine silicon powder is dispersed with a solution of pH≤4.5, the uranium (U) element content in the purified silicon powder can be reduced to 7.3×10-9.
However, due to the failure to remove the hexavalent uranium element dispersed in the acid slurry, the uranium element is re-adsorbed on the surface of the silicon micropowder particles during the sedimentation and drying process, which reduces the purification effect. uranium element. After using the mesoporous adsorption device, the hexavalent uranium (U6+) element dispersed in the slurry could be effectively adsorbed by the SiO2-based aerogel and decreased gradually with the increase of adsorption times. After three purification and separation experiments, The uranium (U) element content can be reduced to 6×10-10. This shows that when the purity of the material cannot meet the requirements for the direct production of high-purity and low-radioactive spherical silica powder, the uranium content can also be reduced by using the selective purification technology. The experimental results also show that the uranium in the silicon micropowder can be effectively separated by acid washing, and the separated uranium can be efficiently adsorbed by the mesoporous adsorption material. Based on this process technology, subsequent batch production can be carried out.
2. Spheroidization and particle size distribution design
First, add zirconia ceramic protection to the surface of all parts that the ultrafine silicon powder may come into contact with in the subsequent process to ensure that the uranium (U) element will not be introduced in the subsequent process to cause secondary pollution, and then put the ultrafine silicon powder into the In the spheroidizing furnace, through the temperature field (1800 ~ 2200 ℃), the air flow field (with oxygen as the carrier gas and oxidant, natural gas as the gas, the ratio of the flow rate of the gas and the oxidant is 1.05) and the material flow (50 ~ 500kg/ h) The control of spheroidization is carried out, and the ultra-fine silicon powder stays in the temperature field for 0.1-3s under a certain air pressure. The spheroidized products are subjected to particle size classification and compounding, and the corresponding particle size distribution is designed according to different packaging requirements.
The production process reduces the excessive dependence on high-purity raw materials for the production of high-purity and low-radioactive spherical silica micropowder to a certain extent.
How to modify the surface of nano-zinc oxide?

Nano-zinc oxide is a new type of functional fine inorganic chemical material. Due to its small particle size and large specific surface area, it has unique physical and chemical properties in chemical, optical, biological and electrical aspects. It is widely used in antibacterial additives, catalysts, rubber , dyes, inks, coatings, glass, piezoelectric ceramics, optoelectronics and daily chemicals, etc., the development and utilization of broad prospects.
However, due to the large specific surface area and specific surface energy of nano-zinc oxide, the surface polarity is strong, and it is easy to agglomerate; it is not easy to disperse uniformly in organic media, which greatly limits its nano-effect. Therefore, the dispersion and surface modification of nano-zinc oxide powder has become a necessary treatment method before nano-materials are applied in the matrix.
1. Surface coating modification of nano-zinc oxide
This is the main surface modification method of inorganic fillers or pigments at present. Surfactant is used to cover the surface of particles to give new properties to the surface of particles. Commonly used surface modifiers include silane coupling agent, titanate coupling agent, stearic acid, silicone, etc.
Wang Guohong et al. used sodium laurate to modify the surface of nano-zinc oxide. Under the conditions that the amount of sodium citrate was 15%, the pH value was 6, and the modification time was 1.5h, the lipophilicity of the modified nano-zinc oxide was improved. The chemical degree reaches 79.2%, and it can be well dispersed in methanol and xylene. Zhuang Tao et al. used titanate coupling agent to modify the surface of nano-zinc oxide. When the amount of titanate was 3%, the temperature was 30°C, and the stirring time was 90min, the activation index of nano-zinc oxide could reach 99.83%. When the modified nano-zinc oxide is applied to natural rubber, its tst and t90 are both extended, and the tensile strength, elongation at break, and flexural flexibility are all improved.
2. Mechanochemical modification of nano-zinc oxide
This is a method of using pulverization, friction and other methods to activate the particle surface with mechanical stress to change its surface crystal structure and physicochemical structure. In this method, the molecular lattice is displaced, the internal energy is increased, and the active powder surface reacts and attaches to other substances under the action of external force, so as to achieve the purpose of surface modification.
The stearic acid molecule is chemically bonded on the surface of zinc oxide, the crystal structure of zinc oxide before and after modification is the same, the agglomeration of its particles is reduced, and the secondary particle size is significantly reduced. By measuring the activation index and lipophilicity of the modified samples, the optimal amount of modifier is 10% of the mass of zinc oxide. The surface of zinc oxide is lipophilic and hydrophobic, and has good dispersion performance in organic solvents.
3. Nano-zinc oxide precipitation reaction modification
The method uses organic or inorganic substances to deposit a layer of coating on the surface of particles to change their surface properties.
At present, some breakthroughs have been made in the preparation technology of nano-zinc oxide, and several industrialized manufacturers have been formed in China. However, the surface modification technology and application technology of nano-zinc oxide have not been paid much attention, and the development of its application field has been greatly restricted. Therefore, it is necessary to strengthen the research on the surface modification and application of nano-zinc oxide products, develop high-performance products, and broaden the application fields of products to meet the demand for nano-zinc oxide products in different fields.
Application of Clay Mineral Materials in Uranium-Containing Wastewater Treatment

Clay minerals are usually layered structures with silicon-oxygen tetrahedron sheets and aluminum-oxygen octahedron sheets connected by shared oxygen in different proportions. They have the characteristics of large specific surface area, strong cation exchange capacity, and ability to adsorb heavy metals and organic matter. The adsorption, desorption and precipitation of uranium ions on the variable charge surface can control the migration and enrichment of uranium elements. It is an ideal adsorption material for uranium enrichment adsorption in solution and uranium removal and recycling in wastewater.
1. Kaolin materials
As one of the most important clay minerals in the natural environment, kaolin plays a key role in fixing and delaying the transfer of pollution. In recent years, the starting point of research on the adsorption of uranyl ions on kaolin is based on the existence of functional groups on the surface of kaolin that can react with uranyl ions. The materials with better adsorption properties obtained by kaolin modification are uranium adsorption materials. One of the main research directions in the future.
2. Attapulgite clay materials
Attapulgite has a unique layer-chain crystal structure, slender fibers, porous, large surface area, and good adsorption performance. The Si4+ contained in attapulgite is replaced by Al3+, and the residual negative charge that appears enables it to adsorb heavy metal ions and radionuclides from the aqueous solution.
The naturally occurring attapulgite clay is endowed with unique properties of composite materials through activation or modification treatment, which can be widely used in environmental wastewater treatment, and the removal, enrichment and utilization of radionuclide uranium provides an inexpensive adsorption and separation material.
3. Montmorillonite materials
Montmorillonite has the advantages of large expansion, strong ion exchange capacity, and the ability to adsorb a large amount of uranium. However, natural montmorillonite is not efficient in treating uranium-containing wastewater, and its adsorption capacity and adsorption performance can be improved by modification.
4. Halloysite materials
Halloysite is a kind of natural clay mineral with unique structure, environmental friendliness, low cost and easy availability. Using its unique structural characteristics and adsorption characteristics, composite materials with efficient uranium adsorption can be prepared. It has a very large role in the field of uranium adsorption materials. potential.
5. Illite materials
Illite is a stable, high load-carrying capacity, low-cost clay mineral. It is a good adsorbent and can remove heavy metals in solution. Illite is also a useful carrier material, which can reduce the aggregation effect and improve its performance. It is an efficient and excellent uranium adsorption material due to its activity and carrying capacity.
Four major modification technologies of hydrotalcite

Hydrotalcite (Layered Double Hydroxides, LDHs) is a layered inorganic carrier functional material, the interlayer anions are exchangeable, and the quantity and type can be strategically adjusted according to actual needs. The tunable denaturation characteristics of this composition and structure of LDHs make them one of the materials with research potential and application prospects in the fields of industrial catalysis, photoelectrochemistry, drug release, plastic modification, and wastewater treatment.
Because LDHs are highly hydrophilic inorganic substances, and the interlayer spacing of the lamellar structure is small, the compatibility with polymers is poor, and the nano-scale dispersion of LDHs is not easy to achieve. In addition, the exchangeability of anions between LDHs layers makes the modified LDHs have specific functional properties. Therefore, LDHs need to be modified to improve the interfacial properties and expand the application range.
There are many modification methods for LDHs, and the appropriate method can be selected according to the required properties and application fields of synthetic materials. Among them, the most commonly used methods mainly include co-precipitation method, hydrothermal synthesis method, ion exchange method and roasting recovery method.
1. Co-precipitation method
Co-precipitation is the most commonly used method for the synthesis of LDHs. Add the mixed aqueous solution containing a certain proportion of divalent and trivalent metal cations into the alkaline solution, control the pH value of the system, maintain a certain temperature, react under constant and rapid stirring until the solution precipitates, and continue to age the precipitate for a period of time , and then filtered, washed and dried to obtain LDHs solid. Usually nitrates, chlorides, sulfates and carbonates can be used as metal salts, and the commonly used alkalis can be selected from sodium hydroxide, potassium hydroxide and ammonia water. The co-precipitation method has the advantages of simple process method, short synthesis period, easy control of conditions and wide application range. Various compositions and types of LDHs can be prepared by using different anions and cations.
2. Hydrothermal method
In general, the hydrothermal method does not require high temperature treatment, and can control the crystal structure of the product to obtain LDHs with obvious layered structure. The mixture was placed in an autoclave, and at a certain temperature, static reactions of different durations were performed to obtain LDHs.
3. Ion exchange method
The ion exchange method is to exchange the interlayer anions of the existing LDHs with other guest anions to obtain a new type of guest LDHs compound. The number and type of anions between the layers can be adjusted according to the desired properties. The guest anion, exchange medium, pH and reaction time all have a great influence on the ion exchange process.
4. Roasting recovery method
The roasting recovery method is divided into two steps. The LDHs were first calcined at high temperature at 500–800 °C, and the interlayer CO32−, NO3− or other organic anion molecules could be removed after the calcination process. The lamellar structure collapsed to obtain Layered Double Oxides (LDO). Then, according to the memory effect of LDO, it absorbs anions to reconstitute into LDHs in aqueous solution. The advantage of the calcination recovery method is that the desired anionic hydrotalcite can be obtained in a targeted manner, and it can eliminate the competition with organic anions, improve the acid resistance, and be applied in a wider pH range. It should also be considered that too high calcination temperature may destroy the layered structure of hydrotalcite. In addition, attention should be paid to the concentration of anionic media during recovery.


