Understand the 16 major application fields and characteristics of illite

Illite is a potassium-rich 2:1-type dioctahedral silicate mica-type clay mineral with missing interlayers, high content of potassium and aluminum, low iron, fine, and good corrosion resistance and resistance. It has excellent physical and chemical properties such as abrasiveness, fluidity, absorption and heat resistance, and is widely used in chemical fertilizers, rubber and plastics, cosmetics, environmental protection, soil conditioning, ceramics, molecular sieves, construction, papermaking, medicine, food and other fields.
1. Fertilizer industry
(1) Potash fertilizer
(2) New granular fertilizer
2. Plastics and rubber industry
Currently, plastic fillers have attracted widespread attention because of their low temperature, high thermal stability, flame retardancy, and good mechanical strength.
3. Super absorbent composite material
Illite and acrylamide can be used as raw materials to synthesize hybrid materials with adsorption capacity. This composite material not only has good adsorption performance, but also enhances the compatibility with the environment.
4. Cosmetics
Illite has a large cation exchange capacity and small particle size, so it can be used as a cosmetic filler. Illite in cosmetics can absorb skin waste and toxins. Illite can be anti-bacterial, non-toxic and other properties, can reflect ultraviolet rays, so it can play a role in anti-ultraviolet.
5. Environmental protection
With the development of industry, the pollution of soil and water bodies has become more and more serious, and the discharge of heavy metal pollutants in the nuclear industry, especially the pollution of radioisotopes, has become increasingly significant, posing a serious threat to the survival of human beings.
6. Soil conditioner
Illite can also be used as a component of clay minerals in some acidic soils. Illite reacts with NaF solution with pH=4.7. This reaction can improve these acidic soils and increase crop yields.
7. Ceramics
In ancient times, illite was the main natural raw material for making pottery. In the production process of ceramics, the content of clay minerals will have a significant impact on the quality of ceramics. This is because illite is rich in potassium, so the increase in illite content will reduce the melting point of the product, reduce water absorption, and reduce the glass phase. ratio increased.
8. Molecular sieve
In industry, illite is mainly used as adsorbent, catalyst and ion exchanger, in addition, illite also has some applications in solar energy conversion and photochemistry.
9. Construction industry
Illite ore is rich in aluminum, which increases the toughness of the product; it is also rich in potassium, which reduces the temperature at which it is calcined during the preparation of porcelain materials, thereby reducing energy consumption. Bricks fired with illite have better thermal insulation effect and lower price.
10. Paper industry
Illite has good absorption, moderate covering ability and transparency, so it can enhance the effect of use.
11. Medicine
Protein, DNA, etc. can be adsorbed by illite, so illite can be used as a carrier of genes in clinical treatment. Illite can be combined with proteins to form complexes into the organism, and then the proteins will be released under the appropriate environment, so as to achieve the purpose of treating diseases.
12. Flame retardant materials
Illite has good chemical inertness, electrical insulation, heat insulation and other properties, and can be used in the production of flame retardant rubber cables, flame retardant textiles, and flame retardant power cables.
13. Synthetic diamond
Due to the good heat resistance, corrosion resistance, insulation and expansion of illite, a small amount of illite clay mineral can be added when preparing diamond.
14. Oil decolorization
Illite can discolor oil, and the illite after surface modification treatment has strong discoloration performance.
15. Oil drilling mud
The particles of illite are small so that it has good floating ability, good heat resistance and wear resistance, and can be used in the process of drilling wells.
16. Food field
Because the far-infrared rays emitted by natural illite powder can decompose or remove the odor released by various foods, and at the same time can activate the water molecules in the food to keep fresh and prevent oxidation, so the deterioration of the food can be avoided.
Preparation of spherical calcium carbonate by hypergravity reaction crystallization and carbonization
The common forms of calcium carbonate mainly include irregular shape, spindle shape, spherical shape, flake shape and cube shape, etc. Different forms of calcium carbonate have different application fields and functions. , solubility and large specific surface area, etc., have important applications in the fields of plastics, rubber, food and paper making.
At present, the main preparation methods of spherical calcium carbonate are metathesis method and carbonization method. Although the metathesis method can produce spherical calcium carbonate with regular morphology and good dispersion, the raw materials of this method are expensive and a large amount of impurity ions will be introduced, which is not suitable for industrial production. The carbonization method is the most commonly used method in the industry. The traditional carbonization method is mainly divided into the intermittent carbonization method and the continuous spray carbonization method. Although the carbonization method has low cost and can be produced on a large scale, the traditional carbonization method for preparing spherical calcium carbonate has problems such as uneven particle size distribution and low production efficiency.
The hypergravity reaction crystallization method is a new method of preparing nanomaterials, and its essence is to generate huge centrifugal force through high-speed rotation, simulating the environment of hypergravity field. The high-speed rotating packing rotor in the hypergravity reactor beats the liquid into liquid filaments, droplets or liquid films, and the specific surface area of the liquid increases sharply. 1 to 3 orders of magnitude, the micro-mixing and mass transfer processes are greatly enhanced, so the reaction time is shorter than the traditional carbonization method, and the product has the advantages of small particle size, narrow particle size distribution, high product purity, and more regular morphology. . Hypergravity reactors are widely used in the preparation of nanomaterials due to their good micro-mixing and mass transfer effects.
Spherical calcium carbonate is grown from vaterite in most cases, but vaterite, as a thermodynamically unstable crystal form, is difficult to exist stably in a humid environment and aqueous solution, and requires some special methods to obtain it stably. The research shows that the introduction of NH4+ during the carbonization reaction can not only inhibit the formation of calcite during the crystallization process, and facilitate the transformation of the crystal form of calcium carbonate to vaterite, but also the atmosphere of NH4+ can make the generated vaterite exist stably in the solution.
Different from NH4+, acidic amino acids will dissociate in solution and combine with Ca2+ to form a seed crystal template. Under the influence of the seed crystal template, the resulting calcium carbonate will also appear metastable crystal phase, and suitable amino acid The introduction will generate specific functions and modify the morphology during the crystallization of calcium carbonate.
Using inexpensive glutamic acid and ammonium chloride as additives, the controllable preparation of spherical calcium carbonate in a hypergravity field was studied, and the effects of the two additives in the synthesis of calcium carbonate were investigated. The results showed that:
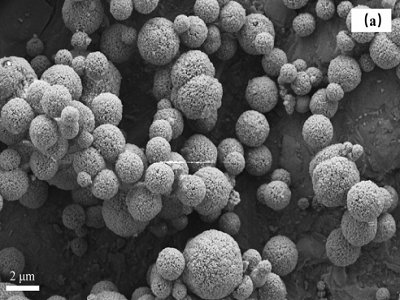
(1) Using the hypergravity reaction crystallization and carbonization method, the particle size can be obtained under the optimal conditions that L-glutamic acid and ammonium chloride are added at 4% and 20% calcium hydroxide, respectively, and the hypergravity factor is 161.0. Pure vaterite calcium carbonate with high sphericity of about 500nm.
(2) Before the reaction starts, L-glutamic acid and calcium ions in the solution form a template, which affects the nucleation and growth of calcium carbonate, and the abundant NH4+ in the solution during the reaction provides a good environment for the formation of vaterite, The high-speed cutting of the liquid by the hypergravity reactor prevents the possibility of excessive coating of calcium hydroxide raw materials, and realizes the controllable preparation of spherical calcium carbonate.
Effect of ultrafine talc on properties of lightweight coated paper

Talc is a magnesium silicate hydrate with a layered structure, with good chemical stability, strong acid and alkali resistance, high whiteness, fine particle size, good dispersibility, stable oil absorption, strong covering power, and electrical properties. Properties such as insulating properties and heat resistance. Talc is rich in resources and low in price. It is one of the most used ultrafine powder products in the world today. It is a promising white pigment and is widely used in ceramics, coatings, paper, textiles, rubber and plastics.
With the deepening of the research on talc powder, the application of talc powder in the paper industry is more and more extensive. Resin adsorbent for slurry when resin problem occurs in the production process of cultural paper and cardboard, and as a pigment for coating to replace part of kaolin or calcium carbonate, it is used to improve the performance of lightweight coated paper and special coated paper, and it is suitable for printing. performance and related ease of operation. The refractive index of talc is comparable to that of kaolin, and it has a flaky crystal form, a high aspect ratio and low oil absorption. It has low hardness and high whiteness. As a white pigment for paper coating, it can not only replace kaolin, but also have some properties better than china clay, especially suitable for light-weight coated paper coatings for rotary printing.
As a kind of paper with one coating and low coating weight, light-weight coated paper puts forward higher requirements for pigment hiding. The widely used kaolin with high hiding in the existing coating formulations. Flake kaolin is mainly imported from Brazil, and the price is relatively high. If a more cost-effective talc powder with the same covering ability and no need to import is used to replace the flake kaolin, the production cost can be continuously reduced on the premise of ensuring product quality, and the cost can be saved. play a positive role.
The effects of superfine talc replacing kaolin on the properties of lightweight coated paper coatings and paper properties were tested. the result shows:
(1) Brazilian kaolin is a thin sheet of clay, and its diameter and thickness are relatively large. Thin sheet china clay is beneficial to improve the coating coverage of light-coated paper, especially the light-coated paper with low coating weight (less than 8g/m2). American kaolin is usually finer in particle size and smaller in diameter and thickness. The high solid content of GCC of Yingge porcelain is conducive to the preparation of high-solids and low-viscosity coatings, and the brightness of the pigments is relatively high. Both superfine talc and Brazilian kaolin are flake structural pigments. The flake particles give the base paper better coverage, which can make the finished paper have better printing properties, such as uniform ink acceptance and high ink retention. The combination of particles of different shapes can obtain a loose coating, which is beneficial to improve the ink absorption of the coating.
(2) After the superfine talc powder replaces the kaolin in the coating formulation, with the increase of the amount of superfine talc powder, the low shear viscosity of the coating tends to increase, but the increase is limited; the water retention of the coating decreases slightly; The shear viscosity showed a decreasing trend, indicating that the use of talc instead of kaolin will have a favorable impact on the coating performance of the coating, which can further increase the solid content of the coating and obtain a better coating effect.
(3) After replacing the kaolin in the formula with ultra-fine talc, with the increase of the amount of ultra-fine talc, the whiteness, smoothness, opacity, gloss, surface roughness, printing gloss, etc. of the lightweight coated paper The quality and performance indicators remained at a similar level, and the printing surface strength was significantly improved.
Influence of particle size of stearic acid modified calcium carbonate on the properties of PBAT composite films

Polybutylene adipate/terephthalate (PBAT) is a copolymer of butylene adipate and butylene terephthalate, which not only has good toughness and stability, but also has excellent Biodegradability is an ideal green environmental protection film packaging material, and it is also one of the most studied biodegradable plastics.
However, the tensile strength of PBAT itself is low, the degradation rate is slow, and the price is 5 to 6 times that of ordinary polypropylene, so it is limited in application and promotion. The current research focuses on how to obtain biodegradable materials with superior performance and low cost. Most of the research is to prepare green composite materials by blending relatively cheap fillers with PBAT, ensuring its degradable properties at the same time. Control costs and expand its application value in the market.
Due to its low price and certain toughening effect on polymers, calcium carbonate is one of the most widely used polymer fillers. Using calcium carbonate as filling powder to prepare PBAT/calcium carbonate composite material has become a feasible way to reduce the cost of PBAT. By studying the properties of PLA/PBAT/nano-calcium carbonate ternary composites, the thermal and physical properties of the composites are greatly improved after adding nano-calcium carbonate. PBAT was filled with calcium carbonate, and it was found that calcium carbonate significantly reduced the cost while improving the mechanical properties of the composite. Modified PBAT with ultrafine calcium carbonate, when adding 20% calcium carbonate, the composite material still has good physical properties.
The surface modification of three kinds of calcium carbonate with different particle sizes was carried out with stearic acid, and the PBAT/modified calcium carbonate composite film was further prepared by melt blending method. The effects of mechanical properties and water vapor transmission properties show that:
(1) Through particle size analysis, the particle size distribution range of activated calcium carbonate is relatively wide, mainly distributed in 1 ~ 20μm, the volume average particle size is 7.6μm; the particle size of ultrafine calcium carbonate is mainly distributed in 0.2 ~ 5μm, volume average particle size. The diameter is 1.5 μm; the particle size distribution of nano-calcium carbonate is relatively concentrated, mainly distributed in 0.2-0.5 μm, and the volume average particle size is 0.34 μm. Through FTIR analysis, it was confirmed that stearic acid has been successfully coated on the surface of calcium carbonate, and the modified calcium carbonate has been dispersed in the PBAT matrix.
(2) After adding modified calcium carbonate, the crystallization temperature, crystallinity and melting temperature of PBAT are increased. When activated calcium carbonate with a volume average particle size of 7.6 μm was added, the crystallization temperature reached a maximum value of 84.12 °C, which was 13.07 °C higher than that of pure PBAT; the crystallinity also reached a maximum, from 10.4% of pure PBAT to 11.48%. When the modified nano-calcium carbonate was added, the melting temperature reached a maximum value of 124.99 °C.
(3) The mechanical properties of PBAT/modified calcium carbonate composite films were significantly improved, and with the decrease of the particle size of modified calcium carbonate, the mechanical properties gradually increased. When the modified nano-calcium carbonate with a volume average particle size of 0.34 μm is added, the tensile strength of the composite film reaches the maximum value of 19.9 MPa, which is 10.07 MPa higher than that of pure PBAT, and the nominal fracture strain reaches 551.8%, which is higher than that of pure PBAT. It is increased by 54%, and the right-angle tear strength is increased from 72.5kN/m of pure PBAT to 139.3kN/m.
(4) The barrier property of the film to water vapor is enhanced after adding modified calcium carbonate. The water vapor transmission rate of the composite film adding activated calcium carbonate is the lowest, which is 232.3g/(m2·24h), which is 28.06 lower than that of pure PBAT film. %, the corresponding water vapor permeability coefficient decreased by 66.09%.
Application of Nano-Calcium Compound Heavy Calcium in the Preparation of Silicone Rubber
![]()
There are many kinds of fillers for silicone sealants, such as silicon dioxide, nano-calcium carbonate, wollastonite powder, heavy calcium carbonate, etc., of which the largest amount is nano-calcium carbonate. In the domestic sealant market, the addition ratio of nano-calcium carbonate in silicone rubber exceeds 60%, and the amount used is very considerable.
More than 70% of the so-called nano calcium carbonate is added with different proportions of heavy calcium carbonate, but it is actually micro-nano composite calcium. Some nano-calcium carbonate synthesis technology is backward, resulting in disordered crystal form (it is difficult to see regular cubes in crystals, mostly small spindles and chain-like mixtures), poor processing performance, and high oil absorption value. Adding heavy calcium carbonate is In order to improve its processing performance, reduce its oil absorption value.
At present, only a few manufacturers can synthesize regular cubic nano-calcium carbonate products, and other irregular nano-calcium carbonate products have poor thixotropy, low tensile strength, low elongation, and poor elastic recovery. , the only benefit is the low price.
These micro-nano composite calcium seem to be cheap, but there are many hidden dangers:
1) poor mechanical properties;
2) The original nano-calcium carbonate has poor crystal form, high surface porosity and high water content, which will lead to poor storage stability or even thickening of the alcohol-based glue;
3) Heavy calcium carbonate is originally a very stable product, and it is mixed with nano calcium carbonate through surface treatment and subsequent drying process, which increases its instability;
4) Nano calcium carbonate is mixed with heavy calcium carbonate, which increases the mixing cost, drying cost and transportation cost of heavy calcium carbonate. It seems to be cheap, but it is actually more expensive.
Compared with the seemingly cheap micro-nano composite calcium, silicone rubber manufacturers use pure nano-calcium carbonate and heavy calcium carbonate in their respective production lines, and the silicone rubber products produced are more stable in performance and lower in cost.
Select pure nano calcium carbonate products with different particle sizes (15nm, 30nm, 40nm, 50nm, 60nm, 70nm) and 1500 mesh inactive heavy calcium carbonate in different proportions to prepare silicone sealants. By comparing the viscosity of the base materials , consistency, extrusion rate and the density, viscosity, consistency, extrusion rate, surface drying time, tensile strength, maximum strength elongation, elastic recovery rate and other indicators of sealant products. The results show that:
(1) More heavy calcium carbonate can be compounded with pure nano-calcium carbonate with finer particle size, and the density and various properties of the obtained sealant meet the standard requirements, and the cost is lower.
(2) Whether it is the production process of directly adding micro-nano composite calcium, or the production process of adding pure nano-calcium carbonate to compound heavy calcium carbonate, it is especially critical to select high-quality (regular crystal morphology) nano-calcium carbonate as a reinforcing material. , which is the main factor determining the mechanical properties of the final silicone rubber product.
(3) Compared with the use of micro-nano composite calcium, the use of high-quality pure nano-calcium carbonate compounded with heavy calcium carbonate to produce silicone rubber not only reduces the production cost of silicone rubber, but also helps to improve its mechanical properties; In terms of management and quality control management, it is also conducive to maintaining long-term stability of product performance.
Influence of Calcium Silicate, Talc, Light Calcium Compound Filler on Properties of Wallpaper Base Paper

As an important interior decoration material, wallpaper is favored by more and more consumers. Generally speaking, paper-based wallpaper requires good bulk and air permeability, and can release the moisture of the wall itself without causing the wallpaper to become moldy.
Compared with a single type of filler, the compound filling of attapulgite and calcium carbonate can significantly improve the strength properties of the paper. One of the main reasons.
Different types of mineral fillers can complement each other and cooperate with each other through compounding and filling, so as to optimize the performance of the filled paper.
(1) The addition of light calcium silicate to the compound filler can significantly increase the bulk of the base paper. At a filling amount of 30%, when calcium silicate: light calcium carbonate = 1:2, the bulk of the filled paper will be increased. The thickness is 15.2% higher than that of talcum powder: light calcium carbonate=1:2 compound filler and paper, and it has little effect on the filler retention rate, paper whiteness and tensile index.
(2) With the increase of the filling amount, compared with talc: calcium silicate: light calcium carbonate = 1:1:1 compound type, calcium silicate: light calcium carbonate = 1:2 compound type The increase of the bulk of the handsheet is more obvious, and the whiteness and opacity of the paper are better under the similar ash content of the finished paper. This is mainly because the whiteness and light scattering properties of light calcium are better, so increasing the proportion of light calcium in the compound filler is beneficial to improve the whiteness and opacity of the finished paper.
What effect do impurity elements have on the quality of high-purity quartz products?

The main impurity elements in quartz are Al, Fe, Ca, Mg, Li, Na, K, Ti, B, H. The impurity elements have a great influence on the quality of high-purity quartz products, such as alkali metals, transition metals, Al and P, etc. Element content is a key indicator of high-purity quartz raw materials. The content requirements of impurity elements vary according to the use of the prepared quartz glass, but the general trend is that the lower the better.
(1) Alkali metal elements Li, K, Na
Reduce the service temperature and mechanical strength of quartz glass, and catalyze the crystallization of quartz glass at high temperature, resulting in devitrification and high temperature deformation of quartz glass. Reducing the content of alkali metal elements is beneficial to increase the softening point of the high-purity quartz crucible, enhance the deformation resistance of the quartz crucible, and improve the yield of single crystals.
IOTA standard sand requires the sum of alkali metal elements to be 2.4×10-6, and the high-purity quartz required for process tubes, silicon wafer processing, quartz blocks, and semiconductor crucibles for single crystal silicon requires the sum of <1.4×10-6, CZ Type crucible requires its sum <0.5 × 10-6, and ultra-high-purity quartz sand for 12-inch or larger silicon wafers requires its sum <0.08 × 10-6.
(2) Transition metal elements Cr, Cu, Fe
The quartz glass produces color spots or causes high temperature discoloration of the quartz glass, which affects the light transmittance and reduces the reliability and stability of the instrument. In the application of optical fibers, it will cause microscopic unevenness, increase fiber loss, and even lead to signal distortion. In semiconductor applications, minute amounts of transition metal elements in the product can promote crystal growth.
(3) Al and P
Entering the quartz lattice will produce strong chemical bonds, which will affect the conductivity of quartz products, and at the same time, enhance the crystallization effect of quartz glass and reduce the service life. A small amount of Al will not affect the quality of high-purity quartz products. IOTA standard sand requires Al element content (12~18)×10-6, but a small amount of Al in the optical fiber will reduce the light transmission of quartz glass. The existence of P element will seriously affect the pulling of single crystal silicon, so the high-purity quartz crucible has high requirements for P, and the content of P element is required to be less than 0.04×10-6.
Silica is expected to occupy an important position in the high-end manufacturing field

Silica is a general term for fine powder or ultrafine particles of anhydrous and hydrous silica or silicates. It is a white, non-toxic, amorphous fine powder or granular material, mainly referring to precipitated dioxide. Silicon, fumed silica, ultrafine silica gel and aerogel, etc.
Silica is industrial monosodium glutamate, with excellent properties such as chemical inertness and porous structure, which can meet the needs of high-end manufacturing applications, such as battery separators, chemical opening agents, CMP abrasives, coating matting agents, special tire reinforcements, etc. .
Filling materials for battery separators: The global demand is about 70,000-80,000 tons, with a CAGR of 5% during 2022-2026. Data show that China's lead-acid battery production accounts for about 45% of the world's total. According to the calculation that silica used for battery PE separator accounts for about 0.2%, the domestic demand is about 30,000 tons, and the global demand is about 70,000-80,000 tons. At present, the main domestic manufacturers include Yueda New Materials, Tongsheng Chemical and Evonik Jialian. The market concentration is relatively high, and the production capacity of high-end products is almost exclusively dominated by Evonik. In the future, lithium battery separators have the possibility of theoretical application.
Coating matting agent: 200,000 tons globally, about 50,000 tons domestically, CAGR 5% during 2018-2026. According to statistics from Transparency Market Research, the market size of nano-silica products for matting agents in the Asia-Pacific region in 2020 is about 1.67 billion yuan, and the CAGR from 2018 to 2026 is 5.29%, accounting for 44% of the global proportion. According to the average price of 15,000/ton, the global demand is about 200,000 tons. There are not many domestic companies producing silica products for coatings, such as Lingwei Technology and Beijing Aerospace Side, and some high-end products rely on imports.
Chemical opening agent: 200,000 tons globally, 100,000 tons domestically, CAGR 5% in 2020-2024. Demand for silica blocking agents will continue to increase due to their low cost and high performance in high volume applications. Technavio predicts that the market size CAGR from 2020 to 2024 is expected to be 5.72%, of which 44% of the growth will come from the Asia-Pacific region. According to this calculation, the market size of anti-blocking agents in the Asia-Pacific region in 2020 is about 3.6 billion yuan, about 100,000-150,000 tons, and the demand in other regions of the world is also 10-150,000 tons. At present, the high-end market is monopolized by foreign giants.
High-end tire reinforcement: the global demand is 1.2 million tons, and the domestic demand is about 450,000 tons. With the continuous promotion of green tires around the world, as a special material for "green tires", the consumption of silica has increased simultaneously, which has formed a strong support for the growth of global demand for silica. In 2020, the consumption will reach 485,000 tons, accounting for the domestic precipitation method The total consumption of silica accounted for 37%. In the future, under the background of the increasing penetration rate of green tires, the demand for silica used in high-end tires is expected to maintain a growth of more than 10%. Among them, it is worth paying attention to the domestic replacement of aircraft tires and the improvement of tire performance requirements brought by new energy vehicles. .
CMP polishing material: domestic demand is 10,000 tons. According to TECHET statistics, the global CMP polishing liquid market size in 2021 will be US$1.89 billion, a year-on-year increase of 13%. The domestic CMP polishing liquid market size is about 3 billion yuan, of which abrasive particles are the largest cost. In 2021, the total amount of fumed silica consumed by my country's chemical mechanical polishing industry will be about 5,400 tons, a year-on-year increase of 4.90%. According to SAGSI, the demand in this field will reach 6,600 tons in 2026, and the compound growth rate from 2022 to 2026 will be 4.00%.
From the perspective of price level distribution, pharmaceutical carriers, catalysts, chemical opening agents, coating matting agents, and adsorbents are all markets with higher unit prices, and their production costs are not much different from ordinary silica, so they have a relatively high unit price. With high profit margins, there are better development opportunities in these segments of the market.
Production process of active nano calcium carbonate for high performance PVC pipe

Activated nano-calcium carbonate is used in plastics, rubber and other polymer materials to fill and reinforce, and to improve the mechanical properties of products, increase the amount of fillers under the condition that the performance remains unchanged, reduce the overall cost of products, and improve product quality. market competitiveness. Therefore, nano calcium carbonate is more and more widely used in plastics, rubber, adhesives, inks and other fields, especially in polyvinyl chloride (PVC) products with the largest amount.
In order to meet the needs of producing high-strength, high-elasticity PVC pipes, Xie Zhong et al. used limestone as raw material to generate lime by calcining, and adopted the double-tower continuous carbonization method to produce nano-calcium carbonate. The surface treatment agent composed of coupling agent and other components is used to activate the calcium carbonate, and the nanometer activated calcium carbonate with low oil absorption value, good processing performance and good dispersibility is prepared.
Production process of active nano-calcium
Using limestone as raw material, it is calcined to generate quicklime CaO and CO2. CaO is dissolved in water-produced slaked lime Ca(OH)2. Add crystal form control agent to slaked lime Ca(OH)2 water, and control certain concentration and temperature conditions. After stirring, the kiln exhaust gas (CO2) is introduced, and the reaction generates nano-calcium carbonate (carbonization).
The nanoscale calcium carbonate slurry is heated to a certain temperature, activated (activated) by adding a surface treatment agent, and then the water in the filter cake is removed by a filter press, and then the nanoscale activated calcium carbonate is obtained by air drying, classification and sieving.
Carbonization process: Double-tower continuous carbonization method is adopted, the first jet tower, the second bubble tower, the effective volume of each tower is 30m3. Add Ca(OH)2 slurry (specific gravity: 1.05), the temperature of the slurry is 15~25℃, add 0.2%~0.8% crystal control agent (calculated on the basis of Ca(OH)2 dry basis), pass CO2, control CO2 The concentration is 30%, the carbonization reaction time is 130min, the end point temperature of the carbonization reaction is ≤55℃, the pH value is 8.0, and the air permeability specific surface area is ≥9.5m2/g. If the dry concentration of Ca(OH)2 is too high, the viscosity of the slurry will increase, the coating phenomenon will be serious, and the calcium carbonate particles are easy to agglomerate into large particles, and the calcium carbonate particles are mixed with Ca(OH)2, control the Ca(OH)2 The mass base concentration of 5% to 10% is appropriate.
Activator: Commonly used activators (surface treatment agents) mainly include inorganic treatment agents, fatty acids and their derivatives, resin acids, coupling agents, polymer compounds and vegetable oils. Activated calcium carbonate products for different uses are mainly different from the use of different surface treatment agents. After the selection of active agent varieties and the optimization of the ratio, four kinds of substances including fatty acid, vegetable oil, non-ionic surfactant and coupling agent were finally selected, and the ratio was 3:2:1:0.5.
Activation process: 3-step surface treatment method is adopted, 3 different activators are activated in 3 times, the CaCO3 slurry (3.0t based on CaCO3 dry basis) is pumped into the 30m3 activation tank, the mixer is started, the speed is 280r/min, and then Add activator for activation, add the saponified fatty acid solution, stir for 1h, and complete the first step of activation. Then, the emulsified vegetable oil and monoglyceride solution were added and stirred for 1 h to complete the second step of activation. Then add the emulsified coupling agent solution and stir for 1 h to complete the third step of activation.
The active nano calcium carbonate produced by this process has low oil absorption value, good processing performance and good dispersibility. It is used as a filling and reinforcing agent in the production of PVC drainage pipes. , Longitudinal retraction rate, flat test and other indicators are better than the national standard for PVC pipes. A 30-ton truck is pressed over the drain pipe, and the water pipe is still restored to its original shape, and the product performance is excellent.
Influence of alkali treatment on acid leaching and purification of vein quartz

Vein quartz is one of the important raw materials for purifying and processing high-purity quartz sand, Vein quartz ore contains many associated gangue minerals, such as feldspar, mica, rutile, tourmaline, chlorite, etc., and many inclusion impurities are also formed. For low-grade vein quartz ore, simple acid leaching generally cannot meet the requirements of high-purity quartz, and some quartz particles still contain inclusions.
The roasting-water quenching method can create gaps between quartz and gangue minerals, exposing a large number of inclusions, thereby increasing the chance of contact between reagents and gangue minerals in quartz, and is more conducive to the removal of gangue minerals.
Using the purification process of roasting-water quenching-magnetic separation-flotation-alkaline treatment-acid leaching, the vein quartz samples were subjected to alkali treatment-acid leaching test, and the influence of alkali treatment conditions on the quality of acid leaching concentrate was systematically studied. :
(1) Through the conditional experiment of alkali treatment and acid leaching of vein quartz flotation concentrate samples, it is found that alkali treatment is beneficial to the reduction of Al content in acid leaching concentrate, and the effects of three different alkali treatments are KOH>NH4OH, respectively. >NaOH, among them, the Al content of the acid leaching concentrate treated with KOH alkali solution is the lowest at 253.67μg/g.
(2) Treated with KOH alkali solution, the optimum reaction temperature is 40℃, the optimum concentration is 0.5mol/L, and the optimum reaction time is 4h. Under the optimum conditions, the Al content in the vein quartz flotation concentrate after alkali treatment and acid leaching treatment was 245.49μg/g. However, too high temperature, too long time, and too large alkali concentration are not conducive to the reduction of Al content in the acid leaching concentrate by alkali treatment, and will cause excessive dissolution of quartz, resulting in a relative increase in Al content.
(3) Through scanning electron microscope analysis, the surface of the alkali-treated quartz is very rough, and there are many tiny pores on the cleavage surface, and a large number of cavities are also dissolved on the fracture surface. During the alkaline treatment, the port face of the quartz is more easily corroded by the alkaline solution than the cleavage face. The test analysis shows that the alkali treatment enhances the effect of acid leaching by eroding the quartz surface and reacting with the surface SiO2 to expose the internal gangue minerals.



