What methods can help surface modification of ultrafine powders?

Ultrafine powder, also known as nanopowder, refers to a type of powder whose particle size is in the nanometer range (1~100nm). Ultrafine powder can usually be prepared by ball milling, mechanical crushing, spraying, explosion, chemical deposition and other methods.
Nanopowders have attracted people's attention due to their special properties in terms of magnetism, catalysis, light absorption, thermal resistance and melting point due to their volume effect and surface effect. However, due to their small size and high surface energy, nanoparticles have a tendency to spontaneously agglomerate. The existence of agglomeration will affect the performance of nanopowder materials. In order to improve the dispersion and stability of the powder and make the application range of the material wider, it is necessary to modify the surface of the powder.
There are many methods of surface modification, which can generally be divided into: surface coating modification, surface chemical modification, mechanochemical modification, capsule modification, high-energy modification, and precipitation reaction modification.
Surface coating modification
Surface coating modification means that there is no chemical reaction between the surface modifier and the particle surface. The coating and the particles are connected by physical methods or van der Waals forces. This method is suitable for the surface modification of almost all types of inorganic particles. This method mainly uses inorganic compounds or organic compounds to coat the surface of the particles to weaken the agglomeration of the particles. Moreover, the steric repulsion generated by the coating makes it very difficult for the particles to reunite. Modifiers used for coating modification include surfactants, hyperdispersants, inorganic substances, etc.
Applicable powders: kaolin, graphite, mica, hydrotalcite, vermiculite, rectorite, metal oxides and layered silicates, etc.
Surface chemical modification
Surface chemical modification uses the adsorption or chemical reaction of functional groups in organic molecules on the surface of inorganic powder to modify the particle surface. In addition to surface functional group modification, this method also includes surface modification using free radical reaction, chelation reaction, sol adsorption, etc.
Applicable powders: quartz sand, silica powder, calcium carbonate, kaolin, talc, bentonite, barite, wollastonite, mica, diatomaceous earth, brucite, barium sulfate, dolomite, titanium dioxide, aluminum hydroxide, Various powders such as magnesium hydroxide and aluminum oxide.
Mechanochemical modification
Mechanochemical modification refers to the change of mineral lattice structure, crystal form, etc. through mechanical methods such as crushing, grinding, and friction. The energy in the system increases and the temperature rises, which promotes particle dissolution, thermal decomposition, and free generation. A modification method that uses radicals or ions to enhance the surface activity of minerals and promote the reaction or attachment of minerals and other substances to achieve the purpose of surface modification.
Applicable powders: kaolin, talc, mica, wollastonite, titanium dioxide and other types of powders.
Capsule modification
Capsule modification is a surface modification method that covers the surface of powder particles with a uniform and certain thickness film.
High energy modification method
High-energy modification method is a method that uses plasma or radiation treatment to initiate polymerization reaction to achieve modification.
Precipitation reaction modification
The precipitation reaction method is to add a precipitant to a solution containing powder particles, or add a substance that can trigger the generation of the precipitant in the reaction system, so that the modified ions undergo a precipitation reaction and precipitate on the surface of the particles, thereby coating the particles. Precipitation methods can be mainly divided into direct precipitation methods, uniform precipitation methods, non-uniform nucleation methods, co-precipitation methods, hydrolysis methods, etc.
Applicable powders: titanium dioxide, pearlescent mica, alumina and other inorganic pigments.
Application of jet mill in anticorrosive coatings

Fly ash, also called fly ash, is a powdery waste formed by calcining in boilers.
Fly ash is typically captured from the flue gas by an electrostatic precipitator or other particle filtering device before the flue gas reaches the chimney.
Fly ash is composed of crystals, glass bodies, and residual carbon. It is gray or gray-black and irregular in shape. Most of the particles are microspherical, with a particle size of 0.1 to 300.0 μm, a density of about 2 g/cm3, and a bulk density of 1.0 to 300.0 μm. 1.8 g/cm3, it has a large specific surface area and strong adsorption activity.
Anti-corrosion performance mechanism of fly ash enhanced coatings
Fly ash contains a large number of microbeads and sponge vitreous structures. Moreover, after the microbeads are crushed, that is, after the surface is destroyed, more pore structures and sponge vitreous structures will be exposed, which can increase the specific surface area of the powder. Utilizing these characteristics, it can be used as a filler in other products, thereby making it a better functional filler for coatings. Research shows that ultrafine fly ash, as a paint filler, can combine covering, leveling and wear resistance.
The corrosion resistance of the coating is closely related to the porosity of the coating. Fly ash is added as a filler in the coating. Due to the pozzolanic effect of fly ash, it can fill the pores of the coating to prevent corrosive media from penetrating into the interior of the coating through the anti-corrosion coating.
Fly ash has good mechanical properties. The fly ash/resin composite coating can increase the durability of the coating, prevent local pores due to wear and loss of protection, and greatly extend the service life of the coating.
The addition of conductive polymer not only improves the water-blocking performance of the coating, but also reduces the oxidation rate of the metal. By adding zinc powder or aluminum powder to the anti-corrosion coating, the active material becomes the anode of the corrosion reaction and protects the metal matrix as the cathode.
Application of jet mill in anticorrosive coatings
Different from the traditional mechanical crushing principle, under the action of high-speed airflow, the material is crushed through the impact between its own particles, the impact and shearing effect of the airflow on the material, and the impact, friction and shearing of the material and other parts. In addition to impact force, the crushing force also includes friction and shearing forces. Friction is caused by the friction and grinding motion between the material particles and the inner wall. Of course, this friction and grinding process also occurs between particles. Because the two crushing methods of impact and grinding are mainly suitable for fine crushing of brittle materials, they are especially suitable.
Jet crushing has some special characteristics because it is different from ordinary crushers in terms of crushing methods and principles:
The fineness of the product is uniform. For the airflow crusher, during the crushing process, due to the centrifugal force of the airflow rotation, the coarse and fine particles can be automatically classified.
The average particle size of the crushed materials is fine and can be crushed to sub-micron level;
The production process is continuous, the production capacity is large, and the degree of self-control and automation is high.
Calcite ultrafine powder preparation process flow

Calcite ultrafine powder, as a commonly used non-metallic mineral material, has a wide range of applications in industry and technology. Its preparation process and quality directly affect the performance and market competitiveness of the product. In this article, we will introduce you to the preparation process of calcite ultrafine powder and its price, hoping to provide you with valuable information.
Calcite ultrafine powder preparation process flow
The preparation of calcite ultrafine powder mainly involves the grinding process. The following is the general process flow:
1. Raw material selection
Selecting high-quality calcite ore as raw material is the first step in preparing ultra-fine powder. The quality of raw materials is directly related to the purity and performance of the final product.
2. smash
The selected calcite ore is crushed, usually using jaw crusher, cone crusher and other equipment to crush the original ore into smaller particles.
3. Grinding
After crushing, the particles are further ground using ultra-fine grinding equipment to obtain the required ultra-fine powder. The selection of ultrafine grinding equipment and the adjustment of process parameters have an important impact on the fineness and particle distribution of the product.
4. Grading
The ground calcite powder may have certain particle inhomogeneity. The ultra-fine powder is screened and classified through classification equipment to obtain the required fineness.
5. Packaging
The finally obtained calcite ultrafine powder is packaged through packaging equipment to ensure product quality and facilitate storage, transportation and sales.
Calcite ultrafine powder is an important non-metallic mineral material, and its preparation process and price are crucial to related industries and application fields.
Whether the surface modification effect of silica powder is good or not depends on these points!

Silica powder itself is a polar and hydrophilic substance. It has different interface properties with the polymer matrix and has poor compatibility. It is often difficult to disperse in the base material. Therefore, surface modification of silica powder is usually required. Purposefully change the physical and chemical properties of the surface of silica powder according to the needs of the application, thereby improving its compatibility with organic polymer materials and meeting its dispersion and fluidity requirements in polymer materials.
Factors such as the raw material quality of silica powder, modification process, surface modification method and modifier, modifier dosage, modification process conditions (modification temperature, time, pH and stirring speed) all affect the surface modification effect of silica powder. Among them, surface modification methods and modifiers are the main factors affecting the modification effect.
1. Quality of silica powder raw materials
The type, particle size, specific surface area, surface functional groups and other properties of silica powder directly affect its combination with surface modifiers. The modification effects of different types of silica powder are also different. Among them, spherical silica powder has good fluidity, is easy to combine with the modifier during the modification process, and can be better dispersed in the organic polymer system. And the density, hardness, dielectric constant and other properties are significantly better than the angular silica powder.
2. Surface modification methods and modifiers
At present, the surface modification methods of silica powder are mainly organic modification, inorganic modification and mechanochemical modification, among which the most commonly used modification method is organic modification. When the single modification effect is not good, you can consider combining organic modification with other modification methods for composite modification.
(1) Organic modification
Organic modification is a method that uses functional groups in organic matter to carry out physical adsorption, chemical adsorption and chemical reactions on the surface of silica powder to change the surface properties of silica powder.
(2) Inorganic modification
Inorganic modification refers to coating or compounding metals, inorganic oxides, hydroxides, etc. on the surface of silica powder to give the material new functions. For example, Oyama et al. used a precipitation method to cover the SiO2 surface with Al(OH)3, and then wrapped the modified SiO2 with polydivinylbenzene, which can meet certain special application requirements.
(3) Mechanochemical modification
Mechanochemical modification refers to first using ultra-fine grinding and other strong mechanical forces to activate the surface of powder particles to increase active points or active groups on the surface of silica powder, and then combining modifiers to achieve composite modification of silica powder.
3. Modifier dosage
The amount of modifier is usually related to the number of active points (such as Si-OH) on the surface of silica powder and the monomolecular layer and bimolecular thickness of the modifier covering the surface.
When the amount of modifier is too small, the degree of activation of the surface of the modified silica powder will not be high; when the amount of modifier is too large, it will not only increase the cost of modification, but also form a multi-layer physical layer on the surface of the modified silica powder. Adsorption causes the interface between silica powder and organic polymer to form a weak layer, resulting in the inability to function as a single molecule bridge.
4. Modification process and condition optimization
Commonly used modification processes for silica powder mainly include dry modification, wet modification, and composite modification.
Dry modification is a modification in which silica powder is dispersed in a modification equipment in a relatively dry state and combined with a certain amount of surface modifier at a certain temperature. Dry modification process is simple and has low production cost. It is currently the main method of surface modification of domestic silica powder and is suitable for micron-level silica powder.
In addition, in order to achieve good modification effect of silica powder, the temperature, pH, time, stirring speed and other process conditions during the modification process should be controlled.
Modification temperature is an important condition for the condensation, dehydration and formation of strong covalent bonds between the modifier and silica powder. The modification temperature should not be too high or too low. Too high a temperature will cause the modifier to decompose or volatilize, and too low a temperature will cause the modifier to decompose or volatilize. This will reduce the reaction rate between the modifier and the silica powder, affecting the modification effect.
Learn about black silicon and its applications
![]()
The origin of the name black silicon is that as seen by the human eye, the color is black. Because of the microstructure on the surface, black silicon can absorb nearly 100% of the incident light, and very little light is reflected, so it appears black to the human eye.
The unique optical and semiconductor properties of black silicon materials have brought a wide range of applications to photoelectric sensors (photodetectors, thermal imaging cameras, etc.), such as low-light cameras that work in the visible and near-infrared dual-bands, bringing great benefits to civilian and military applications. Come to many conveniences.
One of the most attractive properties of black silicon is its fairly low reflectivity and wide-angle absorption capabilities over a wide spectral range. The reflectivity of black silicon can usually reach less than 10%, which is very useful for nanocones or nanowires. The special structure of diameter ratio can further reduce the average reflectivity to less than 3% by optimizing process parameters.
With the development of silicon fine processing technology, the microstructure of black silicon has developed from the earliest nanocone structure processed by femtosecond laser to pyramid, hole, nanowire and composite structures.
After years of exploration, various processing systems have been established for black silicon processing methods. Commonly used methods include femtosecond laser method, electrochemical etching method, reactive ion etching method, acid method, alkali method, metal-assisted etching method, etc. method. Each processing method has different microstructure morphology and available optical properties.
At the same time, the definition of black silicon has gradually expanded. It is no longer limited to microstructured silicon processed by femtosecond laser, and the color is not limited to black. As long as it has obvious light trapping ability, it can be called microstructured silicon. It is black silicon material.
By controlling the characteristic structural size of multilayer porous silicon, researchers artificially control changes in its refractive index. The silicon surface has different absorption effects for different light, and ultimately different colors appear under human eyes. This technical solution can be applied to a four-quadrant detector, so that each quadrant exhibits different spectral response characteristics.
As a new material, black silicon has many excellent properties and has been used in many fields, such as extremely high light absorption rate and light sensitivity, which can be used as the absorbing layer of photodetectors; using black silicon's anti-reflection properties and wide angle Characteristics such as absorption can improve device performance such as photoelectric response rate and response spectral range; black silicon's pyramidal structure has excellent field emission characteristics, so it can be used as a field emission material. Black silicon also has excellent photoemission properties. Due to its luminescent properties, it can be used as a photoluminescent material; using the ultra-high specific surface area of black silicon, it can be used as a solid adhesive or heat dissipation structure between silicon materials.
In many applications, black silicon materials have shown their great value in improving the photovoltaic efficiency of industrial crystalline silicon solar cells. With the explosive development of diamond wire cutting silicon wafer technology, the damage layer during silicon wafer cutting has been greatly reduced, and thinner monocrystalline or polycrystalline silicon wafers can also be provided, which has greatly promoted the vigorous development of the photovoltaic industry and improved the performance of devices. Photoelectric conversion efficiency, photovoltaic cells are in urgent need of front surface technology with low reflectivity and wide-angle absorption and structural design with enhanced absorption. Black silicon technology shows natural coupling in the photovoltaic field.
What are the applications of graphene in the field of thermal conductivity?

At present, with the continuous deepening of research, the application of graphene in the field of thermal conductivity has achieved remarkable results, including the formation of graphene films through chemical bonds between sheets, as a filler in thermally conductive composite materials and thermally conductive coatings, and the preparation of graphene. Polyethylene fiber new functional textile materials, etc.
1. Graphene thermal film
Artificial graphite film has been the most ideal choice for thermal conductive films for a long time in the past. It can usually be used as a heat sink in electronic components and is attached to the surface of electronic components that easily generate heat to evenly disperse the heat generated by the heat source. However, since high thermal conductivity graphite films are mainly prepared using the technical route of PI film carbonization-graphitization method, which requires high-quality polyimide films as raw materials, and its research and development and production have high technical barriers, so the industry has always hoped Other alternatives can be found to solve the problem of raw materials being blocked by technology, and graphene thermal conductive film is an ideal alternative.
2. Thermal conductive filler
As a two-dimensional thermally conductive filler, graphene is easier to form a thermally conductive network than granular fillers, and has good application prospects in thermal interface materials and thermally conductive coatings.
a. As a thermal interface material thermally conductive filler
Compared with traditional granular thermally conductive fillers, thermally conductive fillers using graphene as a thermal interface material can not only utilize its ultra-high in-plane thermal conductivity, but its large diameter-to-thickness ratio is also more conducive to the construction of a three-dimensional thermal conductivity network. It has strong advantages in compounding with fillers of other dimensions to improve the thermal conductivity of thermal interface materials.
b. As a filler for heat dissipation coatings
Heat dissipation problem is a big bottleneck restricting the development of lightweight high-performance devices. As a special industrial coating, heat dissipation coating can increase the heat dissipation speed and efficiency of the surface of the object by enhancing the infrared radiation rate of the heat source surface, and reduce the surface temperature of the material. Meet the need for efficient heat dissipation of devices despite space and size constraints.
3. High thermal conductivity graphene fiber functional textiles
High thermal conductivity graphene fiber is a new type of carbon fiber material composed of graphene units assembled and arranged in an orderly manner. It is assembled in an orderly manner using graphene oxide dispersion or functionalized graphene dispersion through wet spinning. . Its main advantage is that it has good mechanical, electrical and thermal properties at the same time, and can be combined with textile technology to produce functional textiles in large quantities through wet spinning.
Currently, the ultra-high thermal conductivity of graphene can be used to produce electric heating clothing that can keep warm and keep out the cold, as well as thermally conductive and cool-feeling textiles. Graphene electric heating clothing mainly uses graphene to convert the energy of the power supply into heat, and then combines the ultra-high thermal conductivity of graphene to evenly transfer heat to the entire body. It can keep the fabric light and soft while providing excellent thermal insulation performance. The thermally conductive and cool-feeling textiles utilize the high thermal conductivity of graphene, which causes rapid heat loss from the skin surface after skin contact with textiles, significantly lowering body temperature and providing people with a more comfortable wearing experience.
Application progress of ball mill in the field of new materials

Since its introduction more than 100 years ago, ball mills have been widely used in industries such as chemical industry, mining, building materials, electric power, medicine and national defense industry. Especially in the fields of complex mineral processing, powder surface modification, powder activation, functional powder synthesis, mechanical alloying, and ultrafine powder preparation, the mechanical ball milling method has a broad research and application market. .
The ball mill has the characteristics of simple structure, continuous operation, strong adaptability, stable performance, suitable for large-scale and easy to realize automatic control. Its crushing ratio can range from 3 to 100. It is suitable for processing various mineral raw materials and wet grinding. And dry grinding can be used as its abrasive method.
Research progress of mechanical ball milling method in the field of new materials
(1) Lithium battery materials
SiOx materials were synthesized by mechanical ball milling in air atmosphere. Used as anode material for lithium-ion batteries, the volume specific capacity of SiOx can reach 1487mAh/cc, which is more than twice that of graphite; its first Coulombic efficiency is higher than that of untreated SiO, up to 66.8%; and it has excellent cycle stability. After 50 cycles at a current density of 200mA/g, the capacity stabilizes at around 1300mAh/g. The results show that SiOx prepared by this method has practical possibility.
(2) Rare earth materials
In terms of rare earth polishing powder, the mechanical ball milling method not only increases the shear force during the chemical reaction, increases the diffusion rate of particles, is conducive to the refinement of reactants and products, but also avoids the introduction of solvents and reduces It eliminates the intermediate precipitation process, reduces the influence of many preparation conditions in the preparation process of polishing powder, and greatly broadens the research scope of polishing materials. In terms of rare earth catalytic materials, the mechanical ball milling method has a simple preparation process and mild conditions, and can process materials in large quantities.
(3) Catalytic materials
In order to change the particle size of TiO2 and improve its photocatalytic performance, Qi Dongli et al. used high-energy ball milling to process TiO2 powder and studied the effect of ball milling time on the micromorphology, crystal structure, Raman spectrum, fluorescence spectrum and photocatalytic performance of the sample. The degradation rate of TiO2 samples after ball milling is higher than that of non-ball milled samples, and the degradation rate of the sample ball milled for 4 hours is the highest, indicating that it has the best photocatalytic performance.
(4) Photovoltaic materials
The chemical reduction-mechanical ball milling method was used to prepare bright flaky silver powder, and the effects of ball milling method, ball milling time and ball milling speed on the parameters and properties of flaky silver powder were studied. The results show that wet ball milling has higher flake formation efficiency, but the flake silver powder prepared by dry ball milling has a larger flake diameter and a brighter silver appearance.
(5) Perovskite materials
Lead-free double perovskite Cs2AgBiBr6 nanopowder was prepared using a mechanical ball milling process. As the ball milling time increases, the Cs2AgBiBr6 nanopowder finally reaches the pure phase, the particle size gradually decreases to about 100nm, and the particle shape changes from rod-shaped to round particles.
(6) Adsorption materials
Non-metallic minerals such as limestone, kaolin, and serpentine are activated through ball milling to strengthen their ability to react with harmful components such as copper, lead, and arsenic in the water phase. This enables an efficient, simple, and low-cost new sewage purification process to be applied to the sewage purification process. Selective precipitation, separation and enrichment recovery of target metal components.
Compared with other methods, during the process of chemical reaction, the ball milling method can significantly reduce the reaction activation energy, reduce the powder particle size, increase the powder activity, improve the particle size distribution, enhance the bonding between interfaces, promote solid ion diffusion and It induces low-temperature chemical reactions to improve the density and optical, electrical, thermal and other properties of the material. The equipment is simple, the process is easy to control, the cost is low, and there is less pollution. It is an energy-saving and efficient material preparation technology that is easy for industrial production.
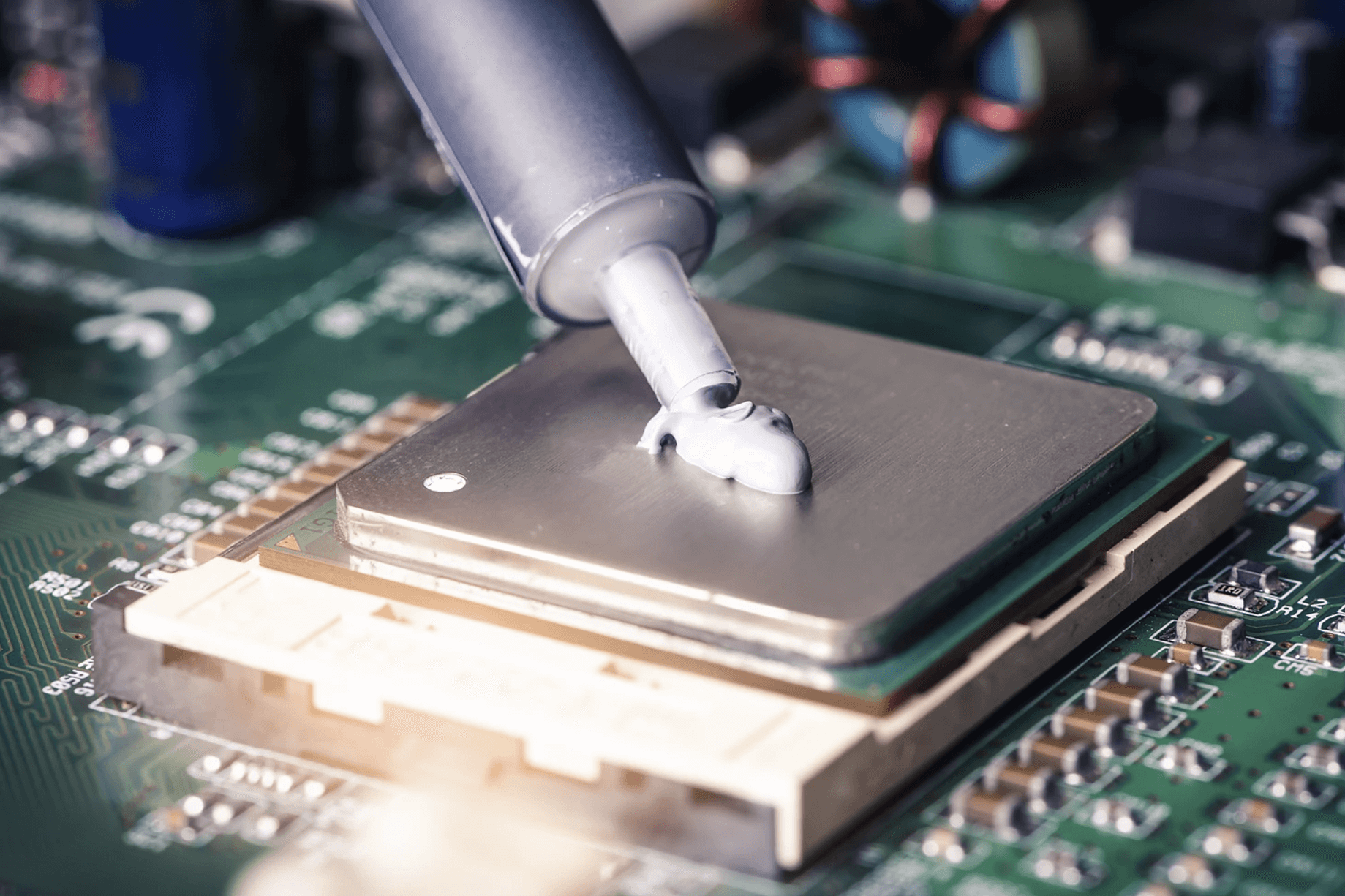
What are the requirements for thermal interface materials in popular application areas?
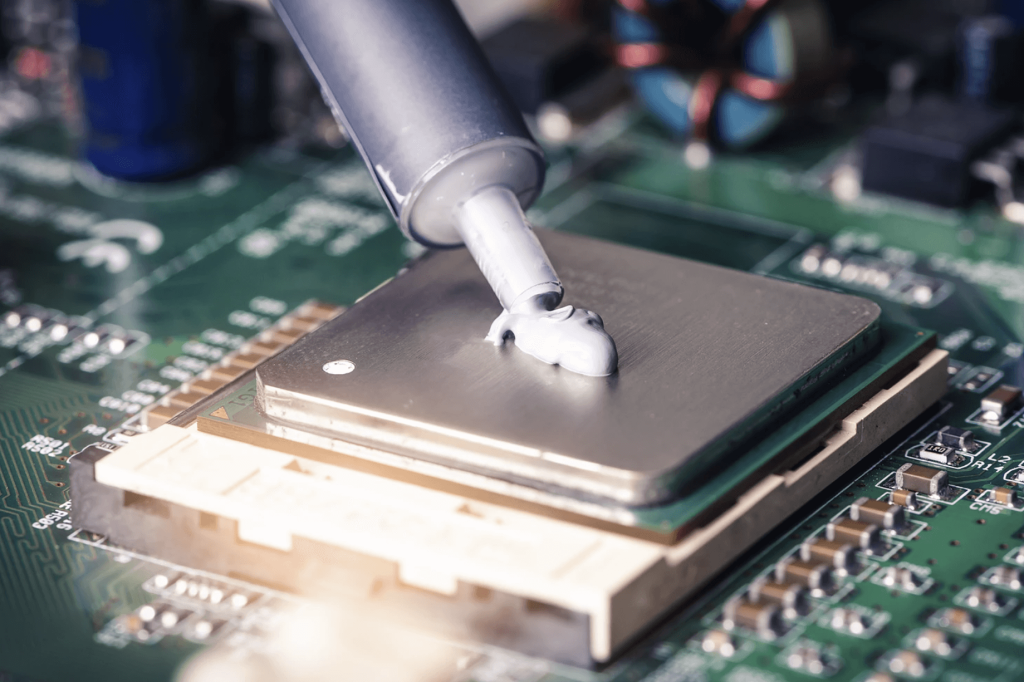
In recent years, the explosion of photovoltaics, electric vehicles, 5G communications and mobile electronics has brought increasingly higher requirements for device heat dissipation. Thermal interface material is a typical thermal conductive material that can be widely coated on heating elements (power tubes, thyristors, electric heating piles, etc.) and radiators (heat sinks, heat sinks, etc.) in various electronic products, power batteries, and electrical equipment.
1. New energy power battery
As the main power source of new energy vehicles, power batteries need to arrange as many battery cells as possible in a certain space to increase their cruising range. This results in a very limited heat dissipation space in the power battery. When the vehicle is running, the heat generated by the battery cells Heat will gradually accumulate in a small heat dissipation space, which will reduce the charging and discharging efficiency of the battery and affect the power of the battery; in serious cases, it will cause thermal runaway and affect the safety and life of the system. Therefore, it is necessary to use thermally conductive potting glue with certain thermal conductivity to achieve potting between battery cells, as well as between the entire battery module group and the heat sink plate. Due to new energy power batteries, the optimal operating temperature range of power battery cells is very narrow, generally between 20-40°C and less than 65°C. To ensure the safety of vehicle operation and optimal battery performance, thermal conductive adhesive is generally required The thermal conductivity of the potting glue reaches more than 3W/(m·K).
2. Photovoltaic inverter
Generally speaking, the thermal conductivity of photovoltaic inverters is required to be no less than 2.0W/mK, and the withstand voltage is no less than 5kV/mm. At the same time, in order to protect the control circuit board and components from the influence of the external environment and mechanical forces, and protect the safety and stability of the circuit, the thermally conductive potting glue used in photovoltaic inverters is also required to have certain earthquake resistance, impact resistance, dust resistance, UV resistance, waterproof and moisture-proof, insulation and other properties. In addition, since the life of photovoltaic systems is generally about 20 years, the life requirements for thermal conductive adhesives used in photovoltaic inverters are also relatively high, usually more than 8 years.
3. 5G base station
The base station is a typical closed natural heat dissipation device. Its heat dissipation method is to allow the heat of the power device to be transferred to the casing first, and then conducted from the casing to the air. Considering the processing properties of electronic equipment in 5G base stations, dispensing technology is often used for construction to improve automation efficiency. Therefore, the thermally conductive adhesive needs to be prepared into a gel state with low stress and high compression modulus.
4. Chip packaging, heat dissipation
Thermal conductive silicone grease with good rheological properties is mainly used for filling between the chip and the packaging shell, and the packaging shell and the heat sink. Since the working temperature of the chip often reaches 60-70°C, the thermal conductivity material used at the chip has very high thermal conductivity requirements. High, it needs to be above 5 W·(m·K), and requires basic properties such as low adhesive layer thickness, high flexibility, high thermal conductivity, low contact thermal resistance, and appropriate thermal expansion coefficient.
The emergence of emerging application fields has put forward more diversified requirements for thermal interface materials, which are no longer limited to improving thermal conductivity, but are developing in the direction of multi-functionality, including dielectric, insulation, high-performance Reliability, flame retardancy and other aspects, so as to better adapt to the specific needs of various fields, thereby promoting technological progress and innovation in related industries.
8 Concepts About Bentonite Clay

1. Bentonite
Bentonite, also known as "bentonite" or "bentonite", is a non-metallic mineral with montmorillonite as the main mineral component. It often contains a small amount of illite, kaolinite, zeolite, feldspar and calcite and other minerals. Montmorillonite The stone content determines the utilization value of natural bentonite.
2. Montmorillonite
Smectite is a large family of minerals with complex chemical composition. The International Clay Association has determined that Smectite is the family name, that is, the smectite family, also known as the smectite family. This group of minerals includes two subgroups, dioctahedral and trioctahedral, and more than a dozen mineral species. Bentonite usually contains minerals from the dioctahedral subgroup, such as montmorillonite, beidellite, nontronite, etc.
3. Sodium bentonite and calcium bentonite
Because part of the silicon ions and aluminum ions in the silicon-oxygen tetrahedron and aluminum-oxygen octahedron are often replaced by other low-priced cations, the montmorillonite crystal structure has a permanent negative charge. In order to balance the electricity price, the montmorillonite unit cell will adsorb exchangeable cations.
According to the type, content and crystallization chemical properties of exchangeable cations contained in bentonite, bentonite is divided into calcium bentonite, sodium bentonite, magnesium bentonite and calcium-sodium bentonite. The most common ones are the first two. .
4. Organic bentonite
Organobentonite refers to using organic ammonium cations to replace exchangeable cations in montmorillonite, covering the surface of montmorillonite, blocking the water adsorption center, causing it to lose its water absorption function, and turning into hydrophobic and lipophilic organobentonite. complex.
Organobentonite can be divided into high-viscosity organobentonite, easily dispersible organobentonite, self-activating organobentonite, and high-purity organobentonite according to functions and components.
5. Lithium bentonite
There are very few natural lithium bentonite resources. Therefore, artificial lithiation is one of the main methods for preparing lithium bentonite.
Lithium bentonite can form gel in organic solvents and replace organic bentonite. Lithium bentonite has excellent swelling, thickening and suspending properties in water, lower alcohols and lower ketones, so it is widely used in architectural coatings, latex paints, casting coatings and other products to replace various organic cellulose suspending agents.
6. Activated clay
Activated clay is made from clay (mainly bentonite) as raw material, which is obtained by inorganic acidification or salt treatment. It is a porous white-off-white powder with a microporous structure and a large specific surface area, and has strong adsorption properties. It is mainly used for decolorization and refining of petroleum processing products (lubricating oil, paraffin, petroleum jelly) and industrial animal and vegetable oils, and is used as adsorbent and catalyst carrier in the chemical industry.
7. Pillared montmorillonite
Pillared montmorillonite is a mineral material with two-dimensional pores formed by polymerized inorganic cations or organic ions (molecules) inserted into montmorillonite. It has a large specific surface area, good thermal stability, strong surface acidity and adjustable pore size. It has broad application prospects in petrochemical industry, sewage treatment, antibacterial materials and other fields.
8. Bentonite gel
Bentonite inorganic gel is a high value-added colloidal product produced with bentonite as the main raw material through purification, sodium modification, phosphating modification and gelation. The preparation process mainly includes the purification of bentonite raw ore, There are four major processes: sodium modification, phosphating modification and gelling.
Inorganic gel is a high value-added bentonite deep processing product that can be used as a thixotropic agent, thickener, dispersant, suspending agent, stabilizer, etc. It is widely used in daily chemicals, pharmaceuticals, detergents, ceramics, glass, papermaking, and casting. , battery and other industries.
Learn more about powders: must-know terms and concepts

Crushing/grinding/pulverizing
The process of reducing particle size.
Dry grinding
The process of crushing in air or other gaseous media.
continuous grinding
The process of continuously and evenly feeding the materials to be processed into the crushing device (or system), and at the same time, the crushed materials are discharged in time.
surface grinding
Under the action of external forces such as friction and shear, the grinding process is mainly based on surface grinding and peeling.
impact grinding
The crushing process is realized by utilizing the impact of the high-speed moving working parts of the crushing equipment on the material or the impact of the high-speed moving material and the wall.
Jet pulverizing
The high-speed jet formed by the expansion and acceleration of compressed gas through the nozzle causes impact, collision and friction between particles and between particles and the wall, thereby realizing the crushing process.
Crushing ratio/ratio of size reduction
The ratio of the characteristic particle diameters of the feed material and the discharge material during the crushing operation indicates the degree to which the particle size of the material is reduced after crushing.
grinding efficiency
The output rate of qualified products per unit energy consumption per unit time.
grinding balance
During the crushing process, the particle size of the powder material no longer continues to decrease and the specific surface area no longer continues to increase.
mechano-chemistry
Structural or physical and chemical changes induced by mechanical forces during the material crushing process.
grinding media
It is an object that is loaded in the mill and uses the impact, collision, shearing, grinding and peeling effects generated during its movement to crush the material.
Grinding aid
Additional additives to improve crushing and grinding efficiency.
Dispersant/dispersing agent
It is an additive that directional adsorbs on the surface of the treated particles to prevent them from aggregating with each other and maintain the stability of the particles within a certain period of time.
classification
The process of dividing a material into two or more particle size distribution levels.
Sieving
The process of grading using sieves.
fluid classification
The process of classifying liquid or gaseous media.
Dry classification/wind classification (dry classification)
The process of classification in air or other gaseous media.
gravity classification
The process of classifying particles based on the difference in their final settling velocity in liquid or gaseous media.
centrifugal classification
The process of grading based on the different trajectories of particles in the centrifugal force field.
Cut size
According to the particle size, the material is divided into coarse and fine particles and the separation limit particle size of the product.
classification efficiency
The degree of separation of coarse and fine grade products during the classification process is usually expressed by the ratio of the mass of the fine-grained material after classification to the mass of the graded material smaller than the cutting particle size. It is a measure of the quality of the grading operation. an important indicator.
surface treatment
A general term for processes such as particle shaping, surface modification, and surface coating.
particle functional design
The process of changing the morphology, structure and characteristics of particles for the purpose of material functionalization.
Particle shape modification
A process that changes the shape of particles.
sphericity
The process of processing irregularly shaped particles into spherical or approximately spherical particles.
Degree of sphericity
The particle shape is close to a sphere.
surface modification
The process of changing the surface properties of particles through the adsorption, reaction, coating or coating of surface modifiers on the particle surface.
wet modification
The process of surface modification of materials in a slurry with a certain solid-liquid ratio or solid content.
Dry modification
The process of surface modification of dry or dried powder materials.
physical coating
The process of surface modification using physical methods.
mechano-chemical modification
The process of surface modification is achieved with the help of strong mechanical force in the crushing process.
encapsulation modification
The process of surface modification by covering the surface of particles with a homogeneous and certain thickness film.
high energy surface modification
The process of surface modification using irradiation or radiation.
Surface modifying agent
Substances that modify the surface of particles.
surface coating
The process of forming inorganic coatings on the surface of particles.