What are the commonly used agents and processes for surface modification of light calcium carbonate?
Light calcium carbonate is made by chemical processing methods. Because its sedimentation volume (2.4-2.8mL/g) is larger than the sedimentation volume (1.1-1.9mL/g) of heavy calcium carbonate produced by mechanical methods. Its chemical formula is CaCO₃, which reacts with all strong acids to form and corresponding calcium salts (such as calcium chloride CaCl2), and at the same time emit carbon dioxide. At temperature (25℃), the concentration product of light calcium carbonate in water is 8.7/1029 and the solubility is 0.0014; the pH value of the light calcium carbonate aqueous solution is 9.5 to 10.2; the PH value of the air-saturated light calcium carbonate aqueous solution 8.0-8.6; Light calcium carbonate is non-toxic, odorless, non-irritating, usually white, with a relative density of 2.7-2.9; the sedimentation volume is above 2.5ml/g, and the specific surface area is about 5㎡/g.
Characteristics of calcium carbonate
White powder or colorless crystal, odorless, tasteless. It is decomposed into calcium oxide and carbon dioxide at 82.5℃. Soluble in dilute acid and emit carbon dioxide, insoluble in alcohol. There are two kinds of crystals, one is orthorhombic aragonite and the other is hexagonal rhombohedral calcite. Calcite is irritating.
a. The particles have regular shapes and can be regarded as monodisperse powders, but they can be in various shapes, such as spindle, cube, needle, chain, sphere, flake and quadrangular column. These different shapes of calcium carbonate can be prepared by controlling the reaction conditions.
b. Particle size distribution is narrow.
c. Particle size is small, the average particle size is generally 1-3μm. To determine the average particle size of light calcium carbonate, the short axis particle size in the triaxial particle size can be used as the representative particle size, and then the median particle size as the average particle size. In addition to the description hereinafter, the average particle size refers to the average minor axis particle size.
Light calcium carbonate has small particle size and high surface energy. Intermolecular forces, electrostatic interactions, hydrogen bonds, oxygen bridges, etc. cause calcium carbonate particles to easily agglomerate, or as a filler, it will affect the actual use effect; In addition, the surface of calcium carbonate is hydrophilic the strong -OH, which is alkaline, is a kind of hydrophilic powder, which is unevenly dispersed in high polymer. Therefore, its surface must be modified in application to reduce surface energy, increase surface active groups, and improve the wettability of the interface with the polymer and the interaction with the polymer.
The physical properties of the polymer are affected by the degree of activation, and the degree of activation is not only related to the modifier, but the key point is whether the calcium carbonate particles are truly dispersed. Therefore, the degree of dispersion of calcium carbonate and the quality of the modification effect directly affect its use value and application fields.
Brief introduction of calcium carbonate surface modification
The surface modification method of calcium carbonate is mainly chemical coating, supplemented by mechanochemistry; the surface modifiers used include stearic acid (salt), titanate coupling agent, aluminate coupling agent, zirconium aluminate acid salt coupling agent and atactic polypropylene, polyethylene wax, etc.
Continuous surface modification process of calcium carbonate
Surface modification should be carried out with the help of equipment. Commonly used surface modification equipment is SLG type continuous powder surface modification machine, high-speed heating mixer, vortex mill and fluidization modification machine.
The main factors affecting the surface modification effect of calcium carbonate are: the variety, dosage and usage of the surface modifier (surface modifier formula); the surface modification temperature and residence time (surface modification process); the surface modification of agents and the degree of dispersion of materials, etc. Among them, the degree of dispersion of surface modifiers and materials mainly depends on the surface modification mills.
1. Commonly used reagents and processes for wet modification
Wet activation is to add an activator to a solvent (such as water), stir the calcium carbonate in it to coat the surface, and finally dry it. This is generally done in light calcium carbonate or nano calcium carbonate manufacturers.
The surface energy of calcium carbonate particles is reduced after the wet modification treatment. Even if the secondary particles are formed after pressure filtration and drying, only soft agglomerates with weak binding force are formed, which effectively avoids the chemical bond oxygen bridges cause hard agglomeration in the dry modification. This method is a traditional calcium carbonate surface treatment method, which is suitable for water-soluble surfactants. The advantages of this method are uniform coating and high production quality. However, certain temperature and conditions need to be controlled for drying. Some surface treatment agents are insoluble in water or easily decomposed in water. The use of other organic agents has cost and safety issues.
(1) Stearic acid (salt) surfactant
Stearic acid (salt) surfactant is one of the commonly used surface treatment agents for calcium carbonate modification. It belongs to anionic surfactants. The structure of a long-chain alkyl group at one end of the molecule is similar to the structure of a polymer. It is a lipophilic group, so it is different from high molecular base material has good compatibility, and the other end is a water-soluble polar group, such as a carboxyl group, which can physically and chemically adsorb on the surface of inorganic fillers such as calcium carbonate.
The specific reaction mechanism of stearic acid (salt) modified calcium carbonate is that under alkaline conditions, ROOH- reacts with Ca2+ and other components to form fatty acid calcium precipitates, which are coated on the surface of calcium carbonate, so that the surface properties of the particles are changed from affinity Water becomes lipophilic.
Yue Linhai and his team reported using sodium stearate saponification solution as a medium to prepare composite calcium carbonate by co-precipitation. Jin Ruidi and his team studied the in-situ modification of calcium carbonate by sodium stearate. In the presence of a modifier, modified calcium carbonate was prepared from calcium hydroxide through carbonization, indicating that the hydrophobicity is due to the combination of sodium stearate in the form of ionic bonds. On the surface of calcium carbonate, insoluble calcium stearate is formed.
(2) Phosphate and condensed phosphoric acid surfactants
Phosphate and other fatty acids (esters) are used for the surface modification of calcium carbonate. After the surface modification of calcium carbonate is carried out by polyphosphate (ADDP) with a special structure, the surface of calcium carbonate particles is hydrophobic and lipophilic. The agglomerated particle size is reduced, and the modified calcium carbonate is filled in the PVC plastic system to significantly improve the processing and mechanical properties of the plastic. Mixed use of stearic acid and sodium dodecylbenzene sulfonate for surface treatment of light calcium carbonate can improve the effect of surface modification.
(3) Quaternary ammonium salt surfactants
The quaternary ammonium salt is a cationic surfactant. Its positively charged end is electrostatically adsorbed on the surface of calcium carbonate, and the other end can be cross-linked with polymers to modify the surface of calcium carbonate.
Zhang Zhihong and others used a new type of cationic surfactant Cetyl dimethyl allyl ammonium chloride (CDAAC) to organically modify calcium carbonate, and the modified product was used as a rubber filler and achieved good results.
2. Commonly used agents and processes for dry modification
The dry modification process is to put the calcium carbonate powder into the high-speed mixer, and then put in the surface modifier. With the help of the mixer and a certain temperature, the modifier can be uniformly adsorbed on the surface of the calcium carbonate particles to achieve the modification effect.
The key technical requirements of the dry modification process are: rapid mixing to facilitate the uniform coating of the coupling agent on the surface of the calcium carbonate particles, a suitable temperature to facilitate the reaction and adsorption, and the drying of the calcium carbonate without moisture to avoid the coupling agent React with water first, not with -OH on the surface of calcium carbonate, which will affect the modification effect.
The surface modifier is generally a coupling agent. The coupling agent modifies the surface of calcium carbonate. The group at one end of the coupling agent can react with the surface of calcium carbonate to form a strong chemical bond. The coupling agent the other end of the polymer can undergo a certain chemical reaction or mechanical entanglement with the organic polymer, thereby closely combining two materials with extremely different properties, calcium carbonate and organic polymer. At present, coupling agents on the market mainly include titanate coupling agents, aluminate coupling agents, borate coupling agents and phosphate coupling agents.
(1) Titanate coupling agent
Shown is the process flow of dry surface coating modification with titanate coupling agent. The modification equipment is a high-speed heating mixer.
In order to improve the uniformity of the interaction between the titanate coupling agent and calcium carbonate, inert solvents such as liquid paraffin (white oil), petroleum ether, transformer oil, absolute ethanol, etc. are generally used for dissolution and dilution.
The amount of titanate coupling agent depends on the particle size and specific surface area of calcium carbonate, generally 0.5%-3.0%. The drying temperature of calcium carbonate should be as low as possible below the flash point of the coupling agent, generally 100-120°C. The titanate coupling agent and the inert solvent are mixed and added to the high-speed mixer in the form of spray or dropwise addition, which can be better dispersed and mixed with the calcium carbonate particles for surface chemical coating.
If continuous surface modification equipment is used, such as SLG continuous powder surface modifier, it is not necessary to pre-dilute the titanate coupling agent with solvent.
The calcium carbonate treated with titanate coupling agent has good compatibility with polymer molecules. At the same time, because the titanate coupling agent can form a molecular bridge between calcium carbonate molecules and polymer molecules, it enhances the interaction between organic polymers or resins and calcium carbonate, and can significantly improve thermoplastic composite materials, etc. The mechanical properties, such as impact strength, tensile strength, bending strength and elongation.
Compared with untreated calcium carbonate filler or stearic acid (salt) treated calcium carbonate, the properties of the modified calcium carbonate coated with titanate coupling agent surface have been significantly improved.
(2) Aluminate coupling agent
Aluminate coupling agents have been widely used in the surface treatment of calcium carbonate and the processing of filled plastic products, such as PVC, PP, PE and filler masterbatch. Studies have shown that light calcium carbonate treated with aluminate can significantly reduce the viscosity of the calcium carbonate/liquid paraffin mixed system, indicating that the modified calcium carbonate has good dispersion in organic media.
In addition, the activated calcium carbonate after surface modification can significantly improve the mechanical properties of the CaCO3/PP (polypropylene) blend system, such as impact strength and toughness.
(3) Compound coupling modification
The calcium carbonate composite coupling system is based on the calcium carbonate coupling agent, combined with other surface treatment agents, crosslinking agents, and processing modifiers for comprehensive technical treatment of the calcium carbonate surface.
The coupling agent and various auxiliary agents in the composite coupling system are described as follows:
Titanate coupling agent.
Stearic acid. The effect of treating calcium carbonate with stearic acid alone is not satisfactory. Using coupling agent alone to treat calcium carbonate has a higher cost. Combining stearic acid and titanate coupling agent can receive a better synergistic effect. The addition of stearic acid basically does not affect the coupling effect of the coupling agent. At the same time, it can also reduce the amount of coupling agent and reduce production costs.
Crosslinking agent bismaleimide. In the composite coupling agent system, the use of cross-linking agent can make the inorganic filler and the matrix resin tightly combined through the cross-linking technology, and further improve the mechanical properties of the composite material. This is difficult to achieve with “Bai Yanhua” or simple titanate coupling agent surface treatment.
Processing modifier-80 resin, etc. Various processing modifiers are mainly polymer compounds. Processing modifiers can significantly improve the melt fluidity, thermal deformation properties and gloss of the product surface of the resin.
In order to coat the surface of all calcium carbonate particles with a layer of coupling agent molecules, the spraying or dripping method can be changed to emulsion dipping, and then filtered, dried, crushed and kneaded with crosslinking agent and other additives at high speed (Mixing), evenly dispersed.
In summary, the main components of the calcium carbonate composite coupling system are calcium carbonate and titanate coupling agent. The titanate coupling agent played a major role. On this basis, adding crosslinking agents, surfactants, processing modifiers, etc. can further enhance the surface activity of calcium carbonate fillers, increase the amount of fillers, and improve the performance of composite materials.
The calcium carbonate filler after compound coupling modification is a white powder with a density of 2.7-2.8g/cm3, a pH value of 7-8, and good hydrophobic properties.
Calcium carbonate treated with coupling agent (including light calcium carbonate and heavy calcium carbonate), in addition to being used as a rigid polyvinyl chloride functional filler, it is also widely used as fillers and pigments for adhesives, inks, coatings, etc.
4. Polymer modification
Surface modification of calcium carbonate with polymers can improve the stability of calcium carbonate in the organic or inorganic phase (system). These polymers include oligomers, high polymers and water-soluble polymers, such as polymethyl methacrylate (PMMA), polyethylene glycol, polyvinyl alcohol, polymaleic acid, polyacrylic acid, alkoxy styrene -Copolymers of styrene sulfonic acid, polypropylene, polyethylene, etc.
The process of coating modified calcium carbonate on the surface of polymer can be divided into two types. The polymer is dissolved in an appropriate solvent, and then the calcium carbonate is surface-modified. When the polymer is gradually adsorbed on the surface of the calcium carbonate particles, the solvent is removed to form a coating. These polymers are adsorbed on the surface of calcium carbonate particles to form a physical and chemical adsorption layer, which can prevent calcium carbonate particles from agglomerating, improve dispersibility, and make calcium carbonate have better dispersion stability in applications.
Master batch filler is a new type of plastic filler. The method is to mix the filler and the resin masterbatch in a certain proportion, add some surfactants, pass through high shear mixing, extrusion, and pelletizing to make the masterbatch filler. This kind of master batch filler has good dispersibility, strong bonding force with resin, uniform melting, high addition amount, low mechanical wear and convenient application. Therefore, it is widely used in straps, woven bags, polyethylene hollow products (pipes, containers, etc.), films, etc. According to the different matrix resins, the commonly used masterbatch fillers mainly include atactic polypropylene calcium carbonate masterbatch (APP masterbatch), polyethylene wax calcium carbonate masterbatch and polyethylene calcium carbonate masterbatch fillers.
APP masterbatch is made of calcium carbonate and random polypropylene as basic raw materials, formulated in a certain proportion, and produced through internal smelting, open refining, and granulation. Calcium carbonate must undergo surface activation treatment before compounding with random polypropylene. The ratio of atactic polypropylene and activated calcium carbonate is generally 1:3-1:10. In order to improve the processing and molding properties of atactic polypropylene, part of isotactic polypropylene or part of polyethylene is generally added during molding. The ratio of atactic polypropylene and activated calcium carbonate determines the surface coating level of calcium carbonate particles, which ultimately affects the product quality of APP masterbatch.
In the APP master batch system, the calcium carbonate particles are covered by atactic polypropylene, that is, the calcium carbonate particles are evenly dispersed in the random polypropylene base material. Assuming that the calcium carbonate particles are standard cubic or spherical particles with side lengths or diameters of 10μm, 50μm, and 100μm, respectively, the mass ratio of random polypropylene and calcium carbonate can be used to calculate the surface of each calcium carbonate particle coated with random poly the average imaginary thickness of acrylic. In theory, the more calcium carbonate filled, the better, that is, the smaller the imaginary thickness, the better. But the actual thickness depends on the process equipment and operating conditions.
Using polyethylene wax or polyethylene instead of random polypropylene as the base material and active calcium carbonate filling compound can prepare polyethylene wax calcium carbonate master batch filler and polyethylene calcium carbonate master batch filler.
5. Plasma and radiation modification
Using an inductively coupled glow discharge plasma system and using a mixture of argon (Ar) and high-purity propylene (C3H6) as the plasma treatment gas to modify the heavy calcium carbonate (1250 mesh) powder by low-temperature plasma. The results show that the Ar- Calcium carbonate filler treated with C3H6 mixed gas has good interface adhesion with polypropylene (PP). This is because there is a non-polar organic layer on the surface of the modified calcium carbonate particles, which reduces the polarity of the surface of the calcium carbonate particles and improves the compatibility and affinity with polypropylene (PP).
6. Inorganic surface modification
Condensed phosphoric acid (metaphosphoric acid or pyrophosphoric acid) is used to modify the surface of calcium carbonate powder, which can overcome the disadvantages of poor acid resistance and high surface pH of calcium carbonate powder. The pH of the modified product is 5.0-8.0 (1.0-5.0 lower than before surface treatment), it is hardly soluble in weak acids such as acetic acid, and has better acid resistance.
In addition, zinc sulfate and water glass are added in the calcium carbonate carbonization process for surface modification. When the resulting product is applied to styrene butadiene rubber, its elongation and tear strength can be improved.
The dry modification process is simple, the investment in production equipment and production costs are low, and it can be packaged directly after discharging. However, compared with the wet method, the activation degree is not good, and it is difficult to uniformize the primary particle size of calcium carbonate particles. Therefore, the dry activation process is currently suitable for filler-grade calcium carbonate modification treatment, and it needs to be further improved for functional nano-calcium carbonate.
3. Evaluation of modification effect of calcium carbonate
The evaluation of the effect of modified calcium carbonate can be roughly divided into two categories: direct method and indirect method. The indirect method is to combine the modified calcium carbonate filler with the application system to determine the application performance of the application system. Direct method refers to the determination of surface physical and chemical properties of modified calcium carbonate, such as activation degree, specific surface area, oil absorption value, coating amount, surface structure, and morphology.
(1) Degree of activation
Inorganic fillers generally have a relatively high density and have a hydrophilic surface, which naturally settles in water, while the surface of inorganic fillers treated with surface modification changes from hydrophilic to hydrophobic. This kind of hydrophobic fine particles floats in water without sinking due to the huge surface tension. According to this phenomenon, the concept of activation degree is proposed, which is represented by ω.
ω=weight of floating part in sample (g)/total weight of sample (g)
The change process of ω from 0-100% reflects the degree of surface activation of modified calcium carbonate from small to large.
The test method is as follows, weigh about 5g sample, accurate to 0.01g, add 200ml of water to a 250ml separatory funnel, shake back and forth for 1 min at a speed of 120 times/min, gently place it on the funnel rack, and let it stand for 20 -30min, after the obvious stratification, put the sinking calcium carbonate in a glass sand crucible with a constant weight (accurate to 0.001g) at 105±5℃ in one time, suction and filter the water, and place it in a constant temperature drying box dry to constant weight at 105±5℃, accurate to 0.001g.
(2) Specific surface area
In addition to improving activity, the surface modification process can also effectively prevent secondary agglomeration. Unmodified nano calcium carbonate particles are prone to produce hard agglomerations, and the specific surface area is small. After surface modification, the agglomeration of calcium carbonate particles is greatly improved, and the specific surface area is significantly increased. The larger the specific surface area, the better the dispersion and degree of dispersion of the particles. This is because the surface of the modified nano calcium carbonate particles is coated with a layer of modifier, and the surface energy is reduced, making the particles in a stable state. Even if some particles are agglomerated together, their mutual agglomeration is a soft agglomeration, which is easier to open.
(3) Oil absorption value
The oil absorption value is related to the size, dispersion, degree of aggregation, specific surface area and surface properties of the calcium carbonate particles. Oil absorption value is an important property that affects the practical application of modified calcium carbonate, especially for coatings, plastics, and ink industries. If the oil absorption value is large, the viscosity will increase when used in the coating and ink industry, and the plasticizer consumption will be increased when used in the plastic industry, so the oil absorption value should be low.
Application and technical requirements of nano calcium carbonate in six industries
Nano calcium carbonate is also called superfine calcium carbonate. The name of the standard is superfine calcium carbonate. The most mature industry of nano calcium carbonate is plastic industry, which is mainly used in high-grade plastic products. It can improve the rheological property of plastic masterbatch and improve its formability. As a plastic filler, it has the function of toughening and reinforcing, improving the bending strength and flexural elastic modulus of the plastic, thermal deformation temperature and dimensional stability of the plastic, and also endowing the plastic with thermal hysteresis. Nano calcium carbonate used in ink products shows excellent dispersion and transparency, excellent gloss, and excellent ink absorption and high dryness. Nano calcium carbonate as ink filler in resin based ink has the advantages of good stability, high gloss, no influence on the drying performance of printing ink and strong adaptability.
Nano calcium carbonate is a kind of functional inorganic filler with particle size of 1-100nm. It is widely used in rubber, plastics, papermaking, ink, paint, sealant and adhesive, medicine, toothpaste, food and other fields. However, different applications have different requirements on the particle size, crystal shape, oil absorption value and dispersion of nano calcium carbonate.
1、Application of nano calcium carbonate in plastics
In the processing and production of plastics, ordinary calcium carbonate products can only be used as general fillers. In addition to being used as fillers, modified nano calcium carbonate can also play the role of activator and reinforcing agent, which can increase the volume of plastic products, enhance the hardness and strength of products, improve the processing performance of plastics, and enhance the heat resistance, bending strength and elastic modulus of plastic products And other performance indicators.
Nano calcium carbonate has been widely used in the processing of PVC, PS, PP and other plastics. Among them, the amount of PVC is the largest, especially for wire and cable, pipe and other products. Nano calcium carbonate has a good reinforcement and toughening effect on PVC plastics. Its main nano characteristics make the processed PVC plastics have good mechanical properties such as strength, barrier, flame retardant and thermal stability.
The technical requirements of nano calcium carbonate in plastic industry are as follows:
Oil absorption value: the plastic industry generally requires very low oil absorption value of nano calcium carbonate, because the particle size of nano calcium carbonate is small and the specific surface area is large. If the oil absorption value is large, more plasticizer will be consumed during mixing, which will increase the viscosity of the system, not only affecting the processing performance, but also increasing the production cost.
Crystal shape: mainly cubic, spherical, these crystal products show less flow resistance, easy to produce and process, and do not affect the surface gloss of plastic products.
Particle size: the particle size of nano calcium carbonate used in plastics is generally controlled at about 100nm. If the particle size is too large, it can not reflect the effect of nano calcium carbonate, and will affect the appearance of products; if the particle size is too small, the surface energy will be increased, and the particles will agglomerate seriously, which is difficult to be completely dispersed during processing, resulting in particles on the surface of products.
Dispersibility: nano calcium carbonate with high dispersion should be selected. If nano calcium carbonate agglomerates seriously, the secondary particle size will be much larger than the primary particle size, while the shear force of plastic processing and mixing is not too strong. Some nano calcium carbonate with serious agglomeration is not easy to disperse, which will cause local defects in application and lead to product quality problems.
Moisture: the moisture control should not be higher than 0.5%. If the moisture content is too high, the plastic surface will produce bubbles or hollows.
PH value: the pH value of nano calcium carbonate should be controlled below 10. If the pH value is too high, it will affect the whiteness and surface gloss of the products, and make the appearance worse. At the same time, high pH will also increase the viscosity of the system and affect the processing process.
Among all kinds of non-metallic mineral powder materials used in the plastic industry, the amount of calcium carbonate is the largest, accounting for 60-70% of the total amount of plastic additives. However, there are still many problems in high-performance application research, especially how to solve the agglomeration of nano calcium carbonate, how to improve the dispersion effect of nano calcium carbonate, and how to improve the bonding strength of composite materials have not been effectively solved.
2、Application of nano calcium carbonate in rubber
Nano calcium carbonate is mainly used in tire, wire, cable and rubber products in rubber industry. It can increase volume, reduce cost and improve rubber processing performance. At present, the main calcium carbonate used in rubber is heavy calcium carbonate and ordinary light calcium carbonate. The application field and scope of nano calcium carbonate are also expanding. The rubber products with nano calcium carbonate are much better than ordinary calcium carbonate in elongation, compression deformation, yield resistance and tear resistance. The nano calcium carbonate treated by special technology has high surface activity. Under ultraviolet irradiation, it can release free moving electrons and easily react with oxygen or organic substances to kill viruses and bacteria. Therefore, nano calcium carbonate also has the effect of sterilization and disinfection.
Tire: nano calcium carbonate can partly replace carbon black and white carbon black in the production of automobile tires, but there is still a gap in reinforcement effect. Therefore, it is mainly applied in the parts with less stress, such as sidewall, cord compound, inner layer rubber, buffer rubber, etc. In production, nano calcium carbonate and active zinc oxide can greatly improve the strength of tire tread compound.
Rubber tube and tape: nano calcium carbonate is mainly used to strengthen and whiten the rubber tube and tape, and improve the dispersibility of the rubber compound at the same time.
Wire and cable: nano calcium carbonate is generally used in the protective cover of mine wire and cable, high voltage wire and cable, marine wire and cable, and electrical wire and cable glue.
The technical requirements of nano calcium carbonate in rubber industry are as follows :
Oil absorption value: the rubber industry has higher requirements for the oil absorption value of nano calcium carbonate. The higher the oil absorption value, the better the wettability and reinforcement of rubber.
Crystal form: due to the high reinforcement performance of rubber, the crystal form of nano calcium carbonate should be mainly chain or chain like, and the chain segments will entangle each other during processing, which can enhance the strength of the system
Particle size: the particle size of nano calcium carbonate used in rubber is generally 80-120nm. If the particle size is too large, the reinforcement effect cannot be achieved. However, if the particle size is too small, the contact area between the particle size and the rubber infiltration increases, which makes the dispersion difficult and affects the rubber mixing.
Moisture: the moisture content should not be higher than 0.5%. If the moisture content is too high, the scorching time of vulcanization will be prolonged, which is not conducive to the increase of vulcanization rate.
PH value: the pH value of nano calcium carbonate mainly affects its vulcanization rate, which should be controlled at 9.5-10.5. If the pH value is low, the vulcanization rate will slow down, the efficiency will be reduced and the energy consumption will be increased.
Adding nano calcium carbonate to the rubber can enhance the reinforcing effect of the rubber, and also improve the aging resistance, oil resistance and dispersibility of the material. Compared with ordinary light calcium products, the reinforcing effect of nano calcium carbonate is better, but worse than carbon black and silica. If carbon black and silica are replaced by nano calcium carbonate, the strength of the material will be reduced. If the use amount is too large, the roller sticking phenomenon will occur. Therefore, the technical formula needs reasonable debugging and continuous optimization.
3、Application of nano calcium carbonate in adhesives
The adhesive is mainly composed of base glue, curing agent, filler, coupling agent and catalyst. With the rapid development of China’s real estate, packaging materials, building materials and other fields, the amount of adhesives increases rapidly. As one of the important fillers of adhesives, nano calcium carbonate not only has low price, but also has good compatibility with adhesives. It can accelerate the crosslinking process of adhesives, improve thixotropy, improve the adhesion, tensile strength and reinforcement effect. At present, the application technology of nano calcium carbonate in polysiloxane sealant has been relatively mature, but the application in polyurethane adhesive is still in its infancy. Polyurethane adhesive has excellent adhesion and aging resistance, and has the surface coating property that silicone does not have. In the application field of pollution-free, good adhesion and weather resistance, polyurethane adhesive has obvious advantages.
The main technical requirements of nano calcium carbonate used in adhesives are as follows:
Oil absorption value: oil absorption value is an index that silicone rubber manufacturers pay close attention to, which directly affects the wettability of nano calcium carbonate in the rubber. Higher nano calcium carbonate has advantages in mechanical properties and thixotropy, but it will lead to viscous colloid, consume more additives and increase production cost. The oil absorption value requirements of nano calcium carbonate in formulation systems of different manufacturers are different, which should be determined by It depends on the circumstances.
Crystal form: generally cubic or rhombic hexahedron, and also need to adapt to the technical requirements and production equipment of the product.
If the particle size of CaCO 3 is too small to be controlled, the colloid will be easy to agglomerate; if the particle size is too small, the colloid will be easily agglomerated
Moisture: the lower the moisture content is, the better the nano calcium carbonate is used for adhesives, generally less than 0.5%. If the water content of nano calcium carbonate is higher, the hydroxyl groups on the surface increase, and the aggregates tend to agglomerate with each other, forming a three-dimensional network under the action of the base rubber, which increases the viscosity of the rubber, prolongs the mixing time, reduces the output and increases the energy consumption; too much water also causes the increase of energy consumption It reacts with the additives to produce particles, resulting in poor dispersion of the products and the appearance of particles. In the polyurethane adhesive, there are many isocyanate radicals, which are easy to hydrolyze. The formation of CO2 is the foaming phenomenon on the surface of the product.
PH value: calcium carbonate is a kind of weak alkali salt with pH value of 8-10. The surface coating agent of nano active calcium carbonate is generally weak organic acid or organic acid salt, which has a certain neutralization effect on its surface. In the production process, the phenomenon of calcium carbonate returning to alkali is very common. If the alkali is not properly treated, it will generate water with the acid component in the rubber material, which will hydrolyze siloxane to produce inorganic particles The poor appearance of the product will also affect its mechanical properties.
Specific surface area: as the particle size is controlled at 60 ~ 100nm, the corresponding specific surface area should be controlled at 20 ~ 25m2 / g. if the specific surface area is too large, the reinforcement effect will be enhanced, but at the same time, the extrusion performance of the adhesive will be deteriorated, and the dispersion effect of the product will also be affected.
At present, with the further research of nano calcium carbonate system, it will not play the same role in the field of nano adhesive, such as nano calcium carbonate.
4、Application of nano calcium carbonate in coatings
Heavy calcium carbonate, light calcium carbonate and nano calcium carbonate are widely used in coatings. Compared with heavy calcium carbonate or ordinary light calcium, nano calcium carbonate not only has better reinforcement effect, but also can improve the covering power, gloss, transparency, fast drying property and stability of coatings. In some industries, such as automobile coatings and architectural coatings, nano calcium carbonate can partly or completely replace the expensive titanium dioxide to reduce the cost of enterprises.
The main technology of nano calcium carbonate used in PVC plastisol system is marked with:
Oil absorption value: generally, the requirements are low. If the oil absorption value is high, the viscosity of the system will increase, and more plasticizers will be needed, which will increase the production cost. However, the oil absorption value requirements of nano calcium carbonate for different products are not completely the same, which depends on the specific situation. For example, some customers need products with high oil absorption value, high viscosity and high yield value.
Crystal form: generally cubic
Particle size: generally controlled at 60-100nm. If the particle size is too large, the viscosity of the system will be reduced, the mechanical properties will be affected, and the thixotropy of the system will become worse; if the particle size is too small, nano calcium carbonate will agglomerate seriously, which will easily lead to poor dispersion and pitting on the surface of colloid. At the same time, viscosity and yield value will be increased.
In addition to the above conventional detection indexes, the nano calcium carbonate used in PVC plastisol system also has special requirements for some application properties
It has good thixotropy, i.e. high shear thinning and low shear thickening. When nano calcium carbonate is applied in PVC plastisol system, the viscosity decreases at high shear rate, which is conducive to the flow of coating. However, under the condition of low shear rate before and after construction, the viscosity becomes higher, which can effectively prevent the coating from sagging;
With high yield value, the coating has good strength and can prevent small disturbance and external force impact;
Good quality stability.
At present, there is a big gap in the quality stability of domestic nano calcium carbonate compared with imported products, and some good indicators are difficult to appear and maintain.
5、Application of nano calcium carbonate in ink
The ink is mainly composed of pigments, binders, fillers, additives, etc. the modified nano calcium carbonate has good compatibility with the binder, and has the advantages of high gloss, strong stability, strong adaptability, and does not affect the ink factor and drying performance. It can comprehensively improve the quality of the ink and reduce the production cost.
Nano calcium carbonate used in ink requires high performance. After use, the ink should show good dispersion, absorption, transparency, gloss, good covering power and printing adaptability. The dispersion determines the glossiness, fluidity and transparency of the ink. The crystal shape of nano calcium carbonate is mainly cubic, and the nano calcium carbonate of cube has low oil absorption value, It is characterized by good fluidity and easy dispersion; the particle size is generally between 20 nm and 100 nm; the fluidity is related to the crystal shape and particle size, and the cubic and spherical crystal forms show greater fluidity, while the chain type shows a smaller fluidity. The manufacturers need to select the appropriate nano calcium carbonate according to the type of ink produced; an important index of glossiness ink is the crystal of calcium carbonate The shape is related to the particle size distribution. The nano calcium carbonate of the cube has a narrow particle size distribution, which is arranged orderly in the ink coating, making the printed surface smooth and showing good luster; the whiteness requirement is low, because other pigments need to be added, too high whiteness will cause difficult coloring.
In the ink industry, nano calcium carbonate plays an important role. The quality of the ink determines the quality of the printed matter. The ink prepared with nano calcium carbonate is smooth, stable, good printability and strong covering power.
In the printing process, it also shows good ink absorption, which is conducive to the quick drying of the ink.
6、Application of nano calcium carbonate in Papermaking
In the paper industry, the application of nano calcium carbonate is mainly in the following aspects:
As paper filler, nano calcium carbonate has small and uniform particle size, small wear on equipment, fine and uniform paper products, small particle size, large oil absorption value and specific surface area, which is conducive to the firmness of pigments; good whiteness, high brightness and good light shielding property can improve the whiteness and shading of paper; it can save the amount of pulp used, reduce the cost, and be conducive to environmental protection.
In cigarette paper, the addition of nano calcium carbonate is about 45% – 50%, because of its high refractive index and good opacity, the cut tobacco inside the cigarette paper can not be seen from the outside; when the cigarette is burning, the CO2 released from calcium carbonate can control the burning speed to a certain extent, but not make the smoke extinguish. At the same time, calcium carbonate can keep the ash content after combustion very well It can increase the air permeability of paper and reduce the tar content in cigarette.
In high-grade toilet paper, especially in women’s products, baby products such as sanitary napkins, diapers, diapers and other products, nano calcium carbonate is widely used to produce polyethylene film with good air permeability and water resistance. In addition, due to the small particle size of nano calcium carbonate, the products are delicate, do not hurt the skin, and will not cause sensory discomfort to the human body.
Its application in paper coating. Different from papermaking filler, nano calcium carbonate for coating is mainly transported in the form of slurry. The advantages are saving production energy consumption, reducing cost, no dust and environmental protection. It can be directly pumped into use and simplify the production process. Nano calcium carbonate can improve the glossiness, whiteness, smoothness, surface strength and ink absorption of coated paper due to its high whiteness, large specific surface area, high activity and good reinforcement.
In different products, the requirements of nano calcium carbonate crystal shape are also different. When used in paper-making fillers, they are mainly spindle shaped, chain shaped and spherical; when used in cigarette paper, they are mainly spindle shaped and needle shaped; when used in paper coating, they are mainly spindle shaped, sheet-shaped and cubic.
The application of nano calcium carbonate in papermaking industry still has great development potential. Because there are still many technical bottlenecks and application problems to be solved in the use process, the high-grade nano calcium carbonate products for papermaking still rely on import. However, with the continuous development of papermaking technology, the paper-making process has changed from acid sizing to neutral and alkaline sizing, which provides a good opportunity for the development of calcium carbonate in papermaking, and the application of nano calcium carbonate will become more extensive.
There are many enterprises involved in raw materials, production and application industries in the industrial chain of nano calcium carbonate. In order to realize the integration of the industrial chain, the technical exchange and innovation between relevant enterprises is very important. Only by meeting the demand of supply and demand among various industries and expanding the market can we achieve win-win results.
Source: Fan tiguo. Preparation and application of nano calcium carbonate [D]. Hubei University of technology, 2018
Application of stearic acid in surface modification of nanometer calcium carbonate
There are two major defects in the application of nano-calcium carbonate to organic media: one is that nano-calcium carbonate is an inorganic material with hydrophilic and oleophobic surface. It has poor dispersion in polymers and poor affinity with organisms. It is easy to form agglomerates a, leading to material performance degradation; Secondly, nano-calcium carbonate has small particle size, a large number of surface atoms, large surface energy, strong interaction between particles, which easily forms agglomeration of nano-calcium carbonate powder. As the amount of nano-calcium carbonate used increases, these defects become more obvious, excessive filling will make the material unusable.
Stearic acid is a common long-carbon chain saturated fatty acid. It has both the lipophilic end of the long carbon chain and the hydrophilic end of the carboxyl group. The surface of nano calcium carbonate is hydrophilic, so stearic acid is coated on the nano, the surface of calcium carbonate can greatly improve its lipophilicity. When it is filled in rubber, plastics, advanced inks, its large specific surface area and high specific surface energy are beneficial to the relationship between calcium carbonate particles and organic polymer molecules. The strong bond between them can make the surface of the product bright and have excellent performance.
1. The mechanism of stearic acid coating modified nanometer calcium carbonate
In recent years, studies on coating and modifying nanometer calcium carbonate with stearic acid have also emerged in endlessly.
Chen Yijian et al. explored the formation process of stearic acid (SA) monolayer calcium carbonate crystals at the air-water interface. Using electron microscope and in-situ Brewster angle microscope for testing and characterization, it was observed that under the monolayer of stearic acid, the final calcium carbonate crystals were formed by particle precursor rather than directly derived from solvation. ion. From scanning electron microscopy (SEM) and transmission electron microscopy (TEM), it can be found that the precursor particles are uniform spheres of amorphous calcium carbonate with a diameter of less than 100 nm. The experiment is to produce calcium carbonate through the reaction of Ca(OH)2 and CO2. Amorphous calcium carbonate is produced in the early stage of mineralization, and it exists stably for at least 0.5h. As the quantity increases, amorphous calcium carbonate aggregates to form Calcite phase calcium carbonate.
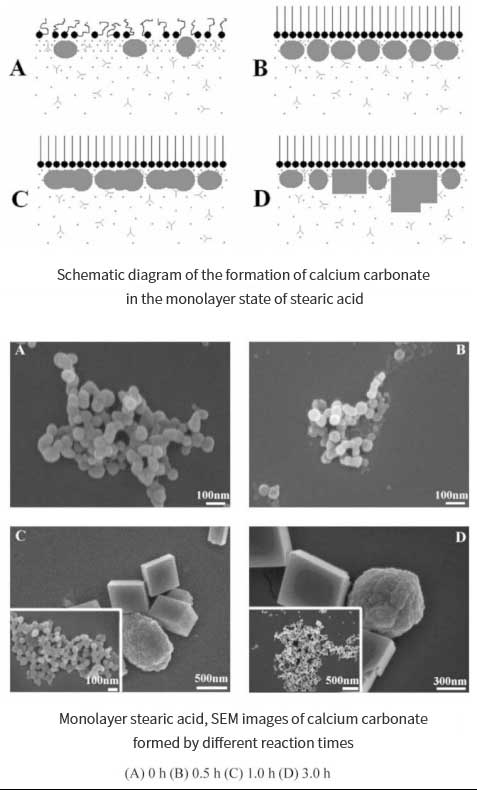
Xuetao Shi et al. used commercial stearic acid to coat precipitated calcium carbonate under water phase conditions, the content of stearic acid in the coated calcium carbonate was 3% to 13.5%. Fourier infrared (FTIR), thermogravimetric (TG) and differential scanning calorimetry (DSC) analysis showed that there is no free stearic acid on the surface of calcium carbonate, only calcium stearate. It is found that the formed calcium stearate is partially chemically adsorbed and partially physically adsorbed on the surface of the coating layer, and can solve the problem that calcium carbonate cannot be fully coated on the surface under water phase conditions. The maximum coating amount is 3.25% .
2. The effect of long-chain fatty acids on calcium carbonate
Long-chain fatty acids also have important effect on the formation of calcium carbonate.
Jiuxin Jiang et al. added various long-chain fatty acids-lauric acid (lauric acid), palmitic acid (hexadecanoic acid) and stearic acid (octadecanoic acid) while blowing carbon dioxide into the calcium hydroxide suspension. Acid) to explore the formation of calcium carbonate. It was found that the addition of long-chain fatty acids did not affect the crystal form of calcium carbonate, but affected the morphology of the calcium carbonate particles produced. When lauric acid is added, the dispersibility of calcium carbonate particles is greatly improved; when a large amount of palmitic acid and stearic acid are added, a microrod-like structure and a spindle-like structure are formed. The author proposes that during the carbonization reaction of calcium hydroxide and carbon dioxide, on the one hand, the length of the carbon chain affects the shape of the micelles formed by the calcium hydroxide suspension, on the other hand, the contact mode between the micelles determines the final formation. The morphology of calcium carbonate.
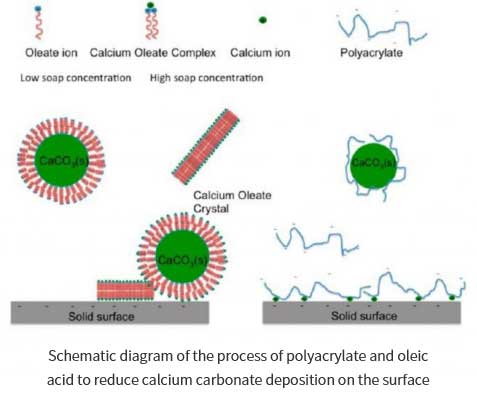
Hao Wang et al. studied the effects of cleaning agents such as polymers, fatty acids, soap liquids on the crystallization, nucleation and sedimentation of active calcium carbonate on hard surfaces (such as stainless steel and silicon surfaces). Thus, on the similar principle, it is instructed how the dishwasher can better remove oil stains during the cleaning process with detergent
3. Application of active nano calcium carbonate
Nano calcium carbonate modified by stearic acid has an important influence as a filler for organic polymers such as silicone resin and polypropylene.
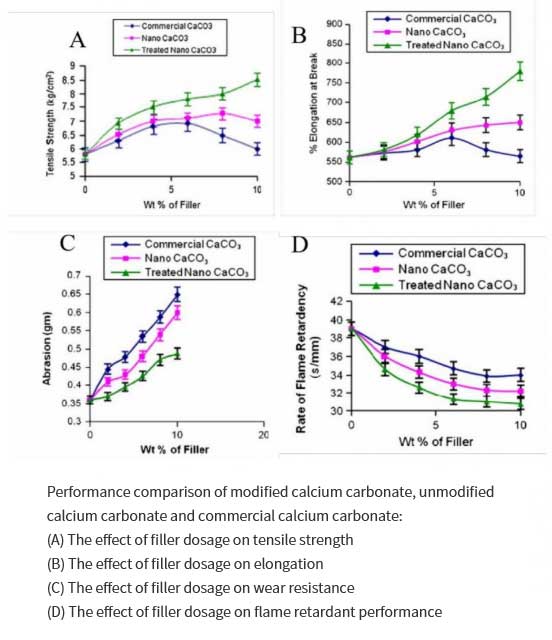
Satyendra Mishra et al. studied the effect of nano-calcium carbonate modified by stearic acid on the properties of silicone resin composites. In the presence of sodium dodecyl sulfonate, they used a certain concentration of CaCl2 and NH4HCO3 to react, filtered and dried to obtain nano calcium carbonate powder. Then in the presence of toluene, a certain amount of stearic acid and nano-calcium carbonate were stirred and mixed to obtain surface-modified nano-calcium carbonate with different stearic acid concentrations, and then added to silicone resin as a filler to improve its performance and obtain modified nano-calcium carbonate. Composite materials, the results show that compared with unmodified nano-calcium carbonate and commercial calcium carbonate, the surface-modified nano-calcium carbonate can greatly improve the tensile strength, elongation, wear resistance and flame retardancy of the composite material. Surface modification can also produce strong adhesion, which makes the polymer chain stronger and improves the thermal stability of the polymer. Based on the high strength and toughness of these nanocomposites, they can be used in cable connectors, electrical and lighting switchgear also of great value in the aerospace field.
Mahdi Rahmani et al. studied the dispersion properties of stearic acid-coated nano-calcium carbonate for polypropylene matrix. TGA was used to analyze the content of stearic acid on the surface of calcium carbonate after the actual coating, and field emission scanning electron microscopy was used to observe the dispersion performance of the sample in the organism after monolayer and multi-layer stearic acid coated nanometer calcium carbonate. The results show that the nano-calcium carbonate modified with stearic acid is filled in the polypropylene organism and can be well dispersed, which reduces the interaction between particles and the adhesion between polymers. After surface modification of stearic acid, nano calcium carbonate eliminates its hydrophilicity and greatly increases the compatibility with the polymer matrix.
As common long-chain fatty acid, stearic acid is cheap and has a wide range of uses and can well modify nano-calcium carbonate. As a cheap and easy-to-obtain filler, activated nano calcium carbonate modified by stearic acid can be well dispersed in many organisms, and can improve the mechanical properties such as tensile strength, elongation, abrasion resistance and flame retardancy of the organism and thermodynamic properties, so choosing stearic acid to modify nanometer calcium carbonate has good research and application value.
Source: Zhou Wei. Surface modification of nanometer calcium carbonate and preparation of hollow rice granular strontium carbonate and hollow fiber barium carbonate[D].
South China University of Technology, 2018.
【Technical analysis】How to choose “industrial monosodium glutamate” calcium carbonate? What is the difference between “heavy calcium” and “light calcium”?
Calcium carbonate is an important and widely used inorganic salt mineral, commonly known as “industrial monosodium glutamate”, is one of the commonly used fillers in all walks of life. Calcium carbonate can not only reduce the raw material cost of rubber and plastic products, but also improve some properties of rubber and plastic materials. Different kinds of calcium carbonate can significantly improve the properties of rubber and plastic materials when used properly. According to the different production process, calcium carbonate can be divided into heavy calcium carbonate and light calcium carbonate.
1. What is the difference between heavy calcium carbonate and light calcium carbonate?
Heavy calcium carbonate and light calcium carbonate have their respective roles in rubber and plastic industry. From an academic point of view, there are many differences between them, such as source, bulk density, pH value, moisture content, crystal shape, oil absorption value, etc. Let’s take a look at the differences between heavy calcium carbonate and light calcium carbonate.
(1) Source:
Heavy calcium carbonate (commonly known as ground calcium carbonate) can be made by directly crushing natural calcite, limestone, chalk and shell by mechanical method (Raymond mill or other high-pressure mill). Because the settling volume of heavy calcium carbonate is smaller than that of light calcium carbonate, it is called heavy calcium carbonate.
Light calcium carbonate, also known as precipitated calcium carbonate, is calcined limestone and other raw materials to produce lime (the main component is calcium oxide) and carbon dioxide, and then water is added to digest lime to produce lime milk (the main component is calcium hydroxide), and then carbon dioxide is added to carbonize lime milk to form calcium carbonate precipitation, and finally dehydration, drying and grinding are carried out. Or it is prepared by the double decomposition reaction of sodium carbonate and calcium chloride to form calcium carbonate precipitate, which is then dehydrated, dried and crushed. Because the settling volume of light calcium carbonate (2.4-2.8 ml / g) is larger than that of heavy calcium carbonate (1.1-1.9 mg / L), it is called light calcium carbonate.
(2) The packing density is different
The most obvious difference between heavy calcium and light calcium lies in the different bulk density of products. The bulk density of heavy calcium products is larger, generally 0.8 ~ 1.3 g/cm³; while the bulk density of light calcium products is small, mostly 0.5 ~ 0.7 g/cm³; the bulk density of some nano calcium carbonate products is even lower, which can reach about 0.28 g/cm³. From the packaging volume of the products, we can roughly distinguish the products of heavy calcium and light calcium. Generally, most of the heavy calcium products are 25kg / package, and the product packaging volume is small, while the light calcium products of the same quality have a larger packaging volume. Some nano calcium carbonate products are also packaged with 15kg / package or 20kg / package.
(3)The whiteness is different
Because there are many impurities in the heavy calcium carbonate product, the whiteness of the product is generally 89% – 93%, and a few products can reach 95%. The light calcium products are made by chemical synthesis, many impurities are removed, and the purity of the products is very high. Therefore, the whiteness of most products is 92% – 95%, and some products can reach 96% – 97%. This is also the main reason why light calcium products are mostly used for filling high-grade or light color products.
(4) The modification function is different
There are slight differences between the modification effects of heavy calcium carbonate and light calcium carbonate. Heavy calcium carbonate is better for tensile strength, while light calcium carbonate is better for impact strength and rigidity. Generally, the surface of plastic with light calcium carbonate is smoother and the density is lower; the processing fluidity of heavy calcium plastic is better, and the properties of plastic filled with small particle size of heavy calcium are also better.
(5) The particle size is different
The particle size of heavy calcium carbonate is 0.5 ~ 45um, and the particle size of product varies with the grinding equipment. The particle size of ordinary light calcium products is generally 0.5-15um, which is difficult to be accurately measured due to its spindle shape, which is generally within a range; the nano calcium carbonate in light calcium is finer, and the size is generally 20-200nm. The particle size of ordinary light calcium carbonate is generally about 2500 mesh, which can meet the performance requirements of PVC pipes and profiles. Therefore, from the perspective of particle size, light calcium carbonate is traditionally used for PVC pipes and profiles. In the past, due to the limitation of crushing equipment, heavy calcium carbonate could not reach this fineness. Now, the particle size of heavy calcium carbonate can fully meet the needs, even finer than light calcium carbonate. Therefore, both PVC pipes and profiles can be selected.
(6) Price difference:
The processing of heavy calcium carbonate is mainly realized by mechanical crushing and grinding; the production of light calcium carbonate is made by chemical reaction precipitation, which is much more complex than heavy calcium carbonate, and the requirements are correspondingly more strict. Therefore, the heavy calcium carbonate with the same particle size is about 30% cheaper than the light calcium carbonate. If the performance allows, the heavy calcium carbonate can be selected, which is more economical and cheaper
2. How to choose calcium carbonate in rubber and plastic industry?
Some people think that the use of foreign plastic products in the filler of calcium is the main position, the classic saying is 14-18:1, so the plastic industry should try to use calcium, instead of light calcium.
The use of heavy calcium and light calcium in plastic products is similar to rubber products. Some manufacturers have reported that under the same conditions, the use of – 400 mesh of heavy calcium instead of light calcium has obvious advantages for products sold by weight, but if the products are sold according to length, area or number, the heavy calcium does not have an advantage over light calcium.
For example, if the same weight of material is filled with the same amount of material, the length of the product obtained will be different. If the tube is filled with heavy calcium, it will be a few thousandths shorter than that filled with light calcium. For artificial leather or synthetic leather measured by area, the difference in area can also be felt. Therefore, plastic product processing plants should not give up the use of light calcium easily.
From an academic point of view, there are many differences between the two, such as different crystal forms, different specific surface areas, different oil absorption values, and so on. In the plastic matrix, the form of the particles of heavy calcium or light calcium is distributed in the matrix macromolecules one by one, or in groups, in the form of loose aggregates in the matrix resin, and the interface state between these particles and resin macromolecules is directly related to the mechanical properties of materials.
It is not the same to use the heavy calcium or the light calcium in the plastic products. We should give full play to their respective advantages and combine the technical and economic factors to make a comprehensive consideration.
For example, in the production of PVC artificial leather, it can be divided into scraping method, calendering method and extrusion method according to the manufacturing process, while the scraping method uses PVC paste resin, which needs to add a lot of plasticizer. The oil absorption value of light calcium is 4-5 times higher than that of heavy calcium. Therefore, the use of light calcium requires more plasticizer to achieve the same flexibility than the use of heavy calcium. If the amount of plasticizer can be reduced, the use of heavy calcium may be more economical.
For example, polypropylene woven bags, woven cloth, packing belt and other unidirectional tensile products, using heavy calcium carbonate and light calcium carbonate as fillers did not find any difference in length. It is found that most of the filler particles are located in the space between the macromolecules formed by stretching. After several times stretching and rapid cooling, the morphology of the macromolecules is rapidly frozen, while the true density of the light calcium and the heavy calcium is almost the same, so the effect on the final length of the product is not obvious. On the other hand, compared with light calcium, the processing fluidity of heavy calcium is better, and the price is much lower. Therefore, in this kind of unidirectional tensile products, it is absolutely dominant.
In addition, in the molding processing technology of plastic door and window profiles, the filler is light calcium, and the dosage is 8-10phr. It should be pointed out that the formula provided by foreign countries is scientific. The starting point of adding calcium carbonate is to improve the overall performance of profile, not to use cheap raw materials to reduce cost.
3. Application of calcium carbonate in degradable plastics
PLA is one of the fastest developing degradable plastics in the market. Calcium carbonate is used as powder filler in raw material processing to provide the performance and price composition of PLA. This method is also widely used in traditional plastics, which can not only improve some properties, but also reduce the cost of plastic raw materials. Compared with other non-metallic mineral powder, calcium carbonate has great advantages: low price, easy coloring, low hardness, less wear for screw and die, good thermal and chemical stability, easy drying, non-toxic and tasteless.
From a technical point of view, it is a long-term process for environmental protection materials to replace non degradable plastics, and the improvement space of degradable plastics in terms of fall resistance, heat resistance and corrosion resistance is another problem. This also means that China’s degradable plastics will usher in development opportunities. By 2030, the demand for degradable plastics in China is expected to reach 4.28 million tons, and the market scale can reach 85.5 billion yuan. Due to the relatively low price, the superfine ground calcium carbonate, light calcium carbonate and nano calcium carbonate can promote the degradation of plastics and are relatively friendly to the environment. In the future, the proportion of the additives in the degradable plastics will be larger and larger, and the market prospect will be more and more broad.
4. Recent situation of calcium carbonate industry in China
There is no doubt that China is rich in calcium carbonate mineral resources. In August, new calcium carbonate deposits were discovered in Guangxi and Hunan, with a total reserve of 607.5 million tons, providing sufficient resources for the development of local calcium carbonate industry.
In recent years, China’s calcium carbonate industry has been in a steady growth trend. In 2019, the output of China’s calcium carbonate industry is 35.95 million tons, including 13.5 million tons of light calcium carbonate and 22.45 million tons of heavy calcium carbonate; the import volume is 49000 tons, the export volume is 122000 tons, and the apparent consumption is 35.877 million tons.
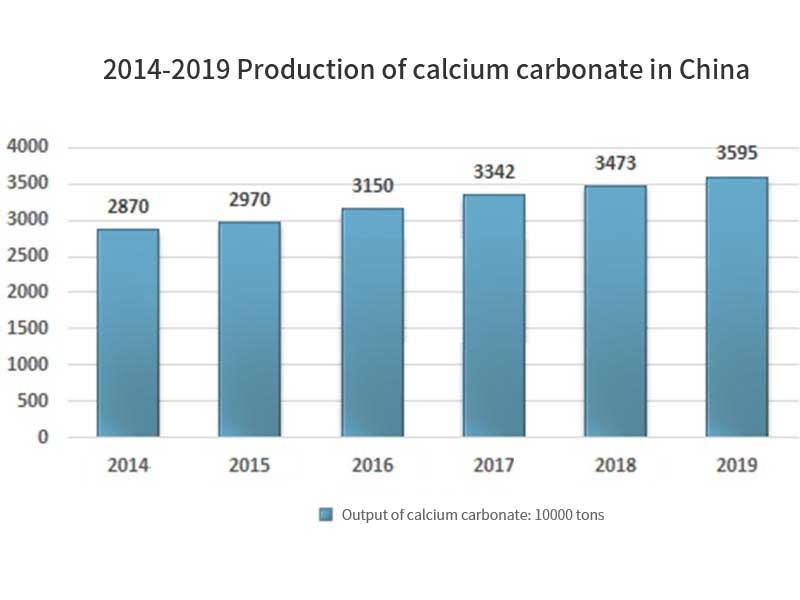
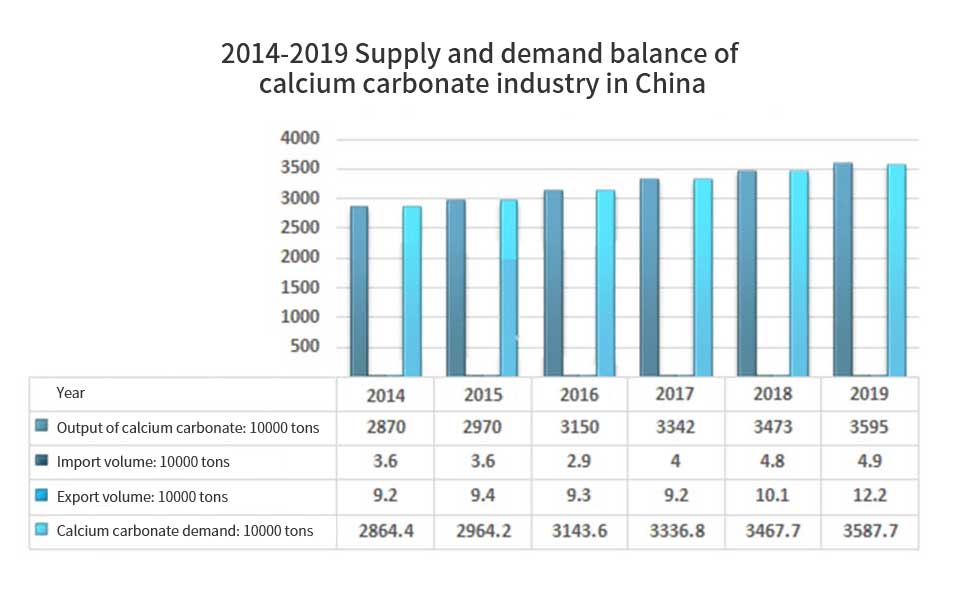
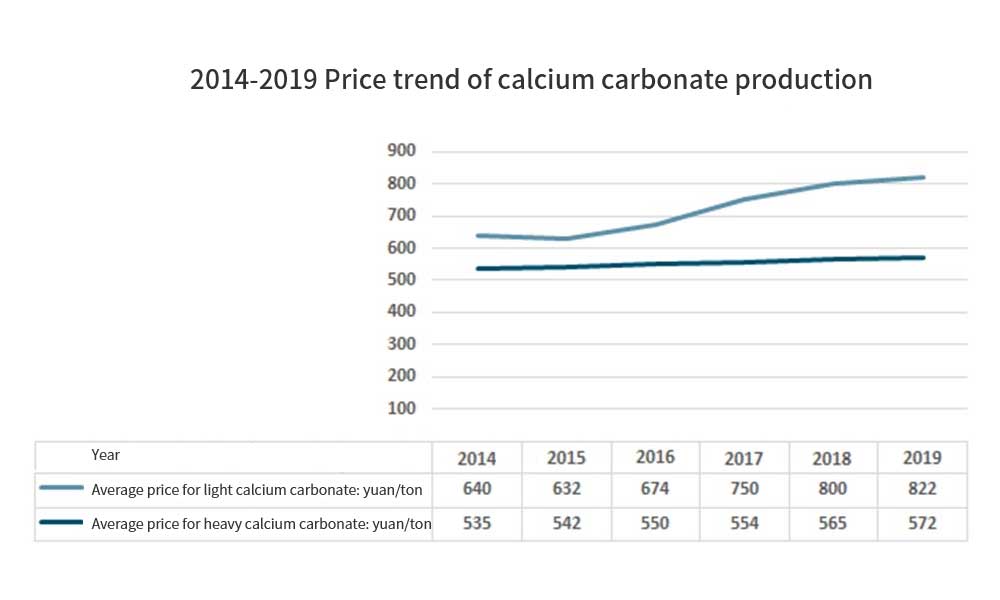
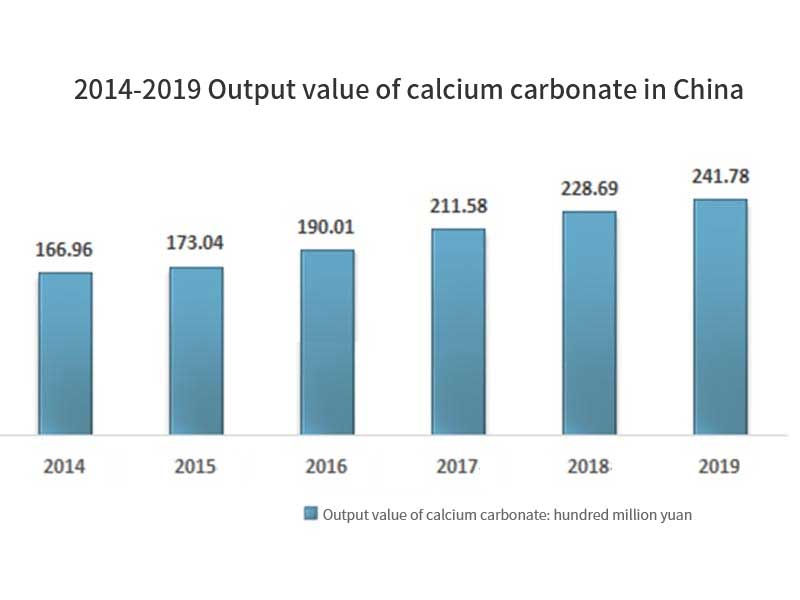
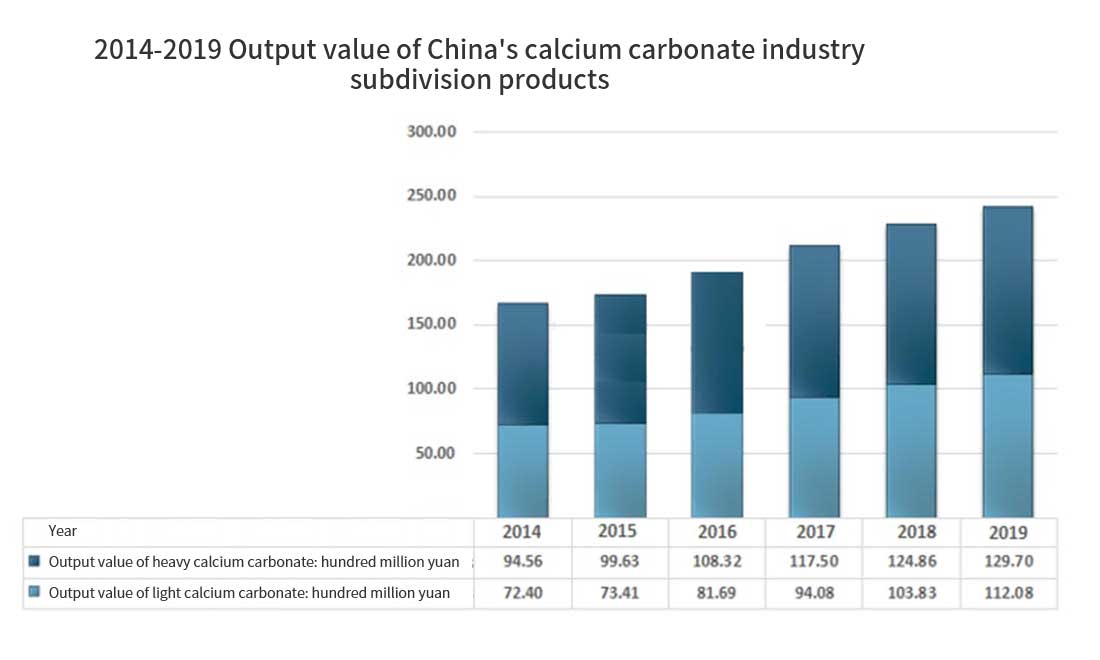
The price of products also shows an increasing trend. Heavy calcium carbonate products increase from 535 yuan / ton in 2014 to 572 yuan / ton in 2019, and light calcium carbonate products increase from 640 yuan / ton in 2014 to 822 yuan / ton in 2019. The output value also maintained a rapid growth, from 16.696 billion yuan in 2014 to 24.178 billion yuan in 2019, with a compound growth rate of 7.69%. In 2019, China’s output value of calcium carbonate is 24.178 billion yuan, including 11.208 billion yuan of light calcium carbonate and 12.97 billion yuan of heavy calcium carbonate.
5. ALPA x Outstanding technology in calcium carbonate industry (Non-metallic minerals)
In the field of non-metallic mineral processing, ALPA can provide the following core technologies:
(1)Large scale and low-cost production can be realized by using ball milling classification process. Taking calcium carbonate as an example, a single production line D97: 10 μm can produce 100000 tons of products annually, and the power consumption per ton of products can reach 150 degrees.Suitable minerals include calcite, marble, limestone, quartz, zircon sand, feldspar, coal gangue, dolomite, magnesite, etc.
(2)The ultra-fine and low-cost production can be realized by using steam mill technology. Taking talc as an example, the particle size of talc can reach 1 μm and the thickness of talc is 300nm. Suitable minerals include talc, graphite, mica, wollastonite, fibrous brucite, attapulgite, kaolin, etc.
(3)The ultra-fine and ultra-pure production can be realized by using Jet mill technology, which is suitable for processing high value-added minerals. Taking quartz as an example, the particle size of the product can reach 2 μm, and the increment of metal impurities in the product is less than 10ppm. Suitable minerals include talc, quartz, barite, graphite, tourmaline, maifanite, etc.
(4)Surface modification technology can meet the application of minerals in rubber and plastic industry, such as Three-roller Mill modification process, Turbo Mill modification process, Pin Mill modification process, High-speed Mixer intermittent modification process, etc. different modification processes and modifiers can be used according to different materials, and the highest coating rate can be achieved with the least modifier. The amount of modifier is about 0.8-1.2%, and the coating rate is about 98%.
Green high value processing concept of non-mineral powder:
(1)The meaning of green :Dry process, no emissions of three wastes; airtight negative pressure, no dust leakage and noise pollution; automation, intelligence and networking; it can realize high-value utilization of solid waste and tailings, recommending with the matched equipment according to requirements. Responding to changes in environmental protection and labor.
(2)The meaning of high value:Keep up with the needs of users for transformation and upgrading, providing with high value-added products based on material science. The research direction focuses on the particle size and its distribution, shape, purity, dispersion and surface modification, determines the processing technology based on its mineralogical composition and structural characteristics, and provides customized solutions combined with environmental protection requirements.
How to select the type of Table roller mill for limestone powder
What is limestone? I believe everyone is familiar with limestone. Limestone can be seen everywhere in our production and life. It has high application value and it’s a common raw material. So what kind of grinding mill can process limestone powder? How to choose? In fact, the table roller mill is a new type of grinding mill that improves the processing efficiency of limestone powder. When formulating the selection and configuration plan, the R&D team needs to combine more milling information to develop a more reasonable configuration plan. Next, let’s check it out.
1. Understand what is limestone
When formulating a selection plan, it is definitely necessary to fully understand the material. Only by grasping its physical and chemical properties, application prospects and other information, can we correspondingly formulate a more reasonable selection and configuration. The main component of limestone is calcium carbonate, which is widely used in the field of building materials. Its main property is that it can decompose calcium oxide and carbon dioxide at high temperature. Therefore, it is an important industrial raw material. When choosing a grinding mill, you need to consider different size, temperature and viscosity of limestone and the model of grinding mill is naturally different, which has become an important parameter for choosing a mill.
2. What is the capacity required by the customer
In fact, this point is very important for the selection. The mill manufacturers have many types of grinding mills, and there are also many types of mills that can grind limestone. Some mills have high productivity but large losses. The other mills have low production capacity but energy saving. For different customers, only suitable ones are better. Therefore, ALPA scientifically customizes the selection and configuration plan, and the production capacity requirements are particularly important.
3. What is the required fineness of the finished product
Anyone who knows the powder industry that different fields have different requirements for powder fineness. Only choosing the right machine to grind the suitable powder fineness is more important for this field. If the fineness of the finished product does not meet the requirements, no matter how large the output is, it will helpless. Therefore, customers need to provide product fineness in time. This requirement is crucial to the selection and configuration of the mill.
After the above detailed narration, everyone should have a better understanding that the three factors, the nature of the material, the production capacity and the fineness of the finished product, all are important references for formulating a suitable selection plan.





